Abstract
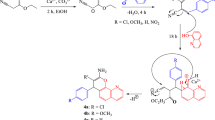
Present communication elicits the designing and synthesis of 3-(1,3-benzothiazol-2-yl) 2-phenyl quinazolin-4(3H)-ones as potential antibacterial agents. A number of substituted 2-amino benzothiazoles, 2-amino-5-[(E)-phenyl diazenyl] benzoic acid, and 2-phenyl-4H benzo[d] [1,3] oxazin-4-one were synthesized as the precursor substrates. The compounds were synthesized in excellent yields and the structures were corroborated on the basis of IR, 1H NMR, Mass, and elemental analysis data. These compounds were screened in vitro for their antibacterial activity against a representative panel of Gram positive and Gram negative bacteria and models were generated through quantitative structure–activity relationship (QSAR).The activity contributions due to structural and substituent effects were determined using sequential regression procedure. The antimicrobial assay data show that the synthesized compounds are found to manifest profound antimicrobial activity.



Similar content being viewed by others
References
Ashcroft AJ, Davies FE, Morgan GJ (2003) Aetiology of bone disease and the role of bisphosphonates in multiple myeloma. Lancet Oncol 4:284–292
Bensimon G, Lacomblez L, Meininger V (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis ALS/Riluzole Study Group. New Engl J Med 330:585
Bertelli L, Biagi G, Giorgi I, Barili PL (2000) Substituted 1,2,3-triazolo[1,5-a]quinazolines: synthesis and binding to benzodiazepine and adenosine receptors. Eur J Med Chem 35:333–341
Dandia A, Singh R (2005) Green chemical multi-component one-pot synthesis of fluorinated 2,3-disubstituted quinazolin-4(3H)-ones under solvent-free conditions and their anti-fungal activity. J Fluor Chem 126:307–312
Foscolos G, Tsatsas G, Champagnac A, Pommier M (1977) Synthesis and pharmacological study of new compounds of benzothiazole. Ann Pharm Fr 35:295
Free SM, Wilson JW (1964) A mathematical contribution to structure–activity studies. J Med Chem 7:395–399
Grover G, Kini SG (2006) Synthesis and evaluation of new quinazolone derivatives of nalidixic acid as potential antibacterial and antifungal agents. Eur J Med Chem 41:256–262
Hansch C, Fujita T (1964) p-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J Am Chem Soc 86:1616–1626
Hays SJ, Rice MJ, Ortwine DF, Johnson G, Schwartz RD, Boyd DK, Copeland LF, Vartanian MG, Boxer PA (1994) Substituted 2-benzothiazolamines as sodium flux inhibitors: quantitative structure–activity relationships and anticonvulsant activity. J Pharm Sci 83:1425
He Y, Benz A, Fu T, Wang M, Covey DF, Zorumski CF, Mennick S (2002) Neuroprotective agent riluzole potentiates postsynaptic GABAA receptor function. Neuropharmacology 42:199
Jimonet P, Audiau F, Barreau M, Blanchard J-C, Boireau A, Bour Y, Coleno M-A, Doble A, Doerflinger G, Huu CD, Donat M-H, Duchesne JM, Ganil P, Gueremy C, Honore E, Just B, Kerphirique R, Gontier S, Hubert P, Laduron PM, Blevec JL, Meunier M, Miquet J-M, Nemecek C, Pasquet M, Piot O, Pratt J, Rataud J, Reibaud M, Stutzmann J-M, Mignani S (1999) Riluzole series. Synthesis and in vivo “antiglutamate” activity of 6-substituted-2-benzothiazolamines and 3-substituted-2-imino-benzothiazolines. J Med Chem 42:2828
Kubinyi H (1993) QSAR: Hansch analysis and related approaches. In: Mannhold R, Krogsgaard-Larsen P, Timmerman H (eds) Methods and principles in medicinal chemistry, vol 1. VCH Publishers, New York, pp 21–137
Kumar V, Mohan C, Mahajan MP (2005) A catalyst- and solvent-free selective approach to biologically important quinazolines and benzo[g]quinazoline. Tetrahedron 61:3533–3538
Malecki N, Caroto P, Rigo B, Goossens JF, Henichart JP (2004) Synthesis of condensed quinolines and quinazolines as DNA ligands. Bioorg Med Chem 12:641–647
Micheal JP (2001) Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 18(5):543–559
Orfi L,Wáczek F, Pató J, Varga I, Hegymegi-Barakonyi B, Houghten RA, Kéri G (2004) Improved, high yield synthesis of 3H-quinazolin-4-ones, the key intermediates of recently developed drugs. Curr Med Chem 11:2549
Paget CJ, Kisner K, Stone RL, Delong DC (1969) Heterocyclic substituted ureas. II. Immunosuppressive and antiviral activity of benzothiazolyl- and benzoxazolylureas. J Med Chem 12:1016
Reddy PS, Reddy PP, Vasantha T (2003) A review on 2-heteryl and heteroalkyl-4(3H)-quinazolinones. Hetrocycles 60:183–226
Sawhney SN, Arora SK, Singh JV, Bansal OP, Singh SP (1978) Synthesis and antiinflammatory activity of 2-amino- and 2-alkylamino-6-benzothiazoleacetic acids, 4-(2′-benzothiazolylamino)-, 4-(4′-substituted-2-thiazolylamino)- and 4-(4′-substituted-3′-alkyl-DELTA.4′-thiazoline-2′-imino)phenylacetic acids. Indian J Chem 16B:605
Sharma P, Sharma S, Rane N (2004) Synthesis and in vitro antimicrobial activities of 2-hydroxy-6-methyl-7-(arylamino)-1,7-dihydropurin-8-ones. Bioorg Med Chem 12:3135
Sharma P, Kumar A, Sharma S, Rane N (2005) Studies on synthesis and evaluation of quantitative structure–activity relationship of 5-[(3′-chloro-4′, 4′-disubstituted-2-oxoazetidinyl)(N-nitro)amino]-6-hydroxy-3-alkyl/aryl[1,3]azaphospholo[1,5-a]pyridin-1-yl-phosphorus dichlorides. Bioorg Med Chem Lett 15:937
Sharma P, Kumar A, Upadhyay S, Sahu V, Singh J (2009) Synthesis and QSAR modeling of 2-acetyl-2-ethoxycarbonyl-1-[4(40-arylazo)-phenyl]-N,N-dimethylaminophenyl aziridines as potential antibacterial agents. Euro J Med Chem 44:251–259
Shirke VG, Bobade R, Bhamaria P, Khadse BG, Sengupta SR (1990) Synthesis and antitubercular activity of some new 2-(substituted arylamino)-5,6-disubstituted/6-substituted benzothiazoles. Indian Drugs 27(6):350
Sielecki IM, Johnson TL, Liu J, Muckelbauer JK, Graftstrom RH, Cox S, Boylan J, Burton CR, Chen H, Smallwood A, Chang C, Boisclair M, Benfield PA, Trainor GL, Seitz SP (2001) Quinazolines as cyclin dependent kinase inhibitors. Bioorg Med Chem Lett 11:1157
Stewart JJP (1989) Optimization of parameters for semi-empirical methods. I: Method. J Comput Chem 10:209
Stewart JJP (1990) MOPAC 6.0. QCPE 455. Indiana University, Bloomington, IN, p 47405
Suter H, Zutter H (1967) Studies concerning benzthiazoles as eventual oral antidiabetics. Helv Chim Acta 50:1084
Witt A, Bergman J (2003) Recent developments in the field of quinazoline chemistry. Curr Org Chem 7:659–677
Wong H, Ganesan A (2000) Total synthesis of the fumiquinazoline alkaloids: solution-phase studies. J Org Chem 65:1022–1030
Valstat software developed at department of pharmacy, SGITS, 23, park road, Indore, India (available on request)
Acknowledgments
We are grateful to Central Drug Research Institute, Lucknow and Defence Research Development Establishment, Gwalior, India for providing spectroanalytical facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P., Kumar, A., Kumari, P. et al. QSAR modeling of synthesized 3-(1,3-benzothiazol-2-yl) 2-phenyl quinazolin-4(3H)-ones as potent antibacterial agents. Med Chem Res 21, 1136–1148 (2012). https://doi.org/10.1007/s00044-011-9626-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9626-0