Abstract
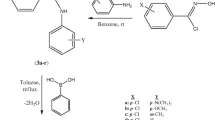
The synthesis and characterization of a series of 4-substituted phenyl-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-thiones were presented. Preliminary in vitro antimicrobial activity of the compounds was assessed against a panel of microorganisms including S. aureus, E. faecalis, P. aeruginosa, E. coli, and C. albicans. Some of the compounds exhibited significantly in vitro antimicrobial activity. The pMIC values were correlated with physicochemical descriptors: Hammett substituent constants (σ m and σ p ) and the lipophilic constant (π). One statistical significant 2D-QSAR model was obtained with para-substituted compounds. The pMIC values were also correlated with some theoretical descriptors as independent variables and four statistical significant 2D-QSAR models were also obtained with meta-substituted compounds.

Similar content being viewed by others
References
Agirbas H, Kemal B, Sagdinc S et al (2009) IR spectroscopic studies on the transmission of substituent effects to carbonyl a nd thiocarbonyl stretching frequencies in 4-substituted phenyl-4,5-dihydrobenzo [f] [1,4] oxazepin-3 (2H)-ones (thiones). Vib Spectrosc 50:307–311
Aono J, Sugawa M, Koide T et al (1991) The role of CGMP in the anti-aggregating properties of by -1949, a novel dibenzoxazepine derivatives. Eur J Pharmacol 195(2):225–231
CLSI (Clinical Laboratory Standarts Institute) (formerly NCCLS) (2002) Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard, CLSI document M27 A2, 2nd ed. CLSI, Wayne: PA, USA
CLSI (Clinical Laboratory Standarts Institute) (formerly NCCLS) (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Approved Std. M-7, A-7, USA
Davion Y, Guillaumet G, Leger J-M et al (2004) Synthesis of substituted 1,4-benzoxazepine-3-one derivatives. Heterocycles 63(5):1093–1112
Derieg ME, Sternbach LH (1966) 4,4-Dihydro-1,4-benzoxazepin-3(2H)-ones. J Heterocycl Chem 3:237–238
Dols PPMA, Folmer BJB, Hamersma H et al (2008) SAR study of 2,3,4,14b-tetrahydro-1Hdibenzo[b,f]pyrido[1,2-d][1,4]oxazepines as progesterone receptor agonists. Bioorg Med Chem Lett 18(4):1461–1467
Draper NR, Smith H (1981) Applied regression analysis, 2nd edn. Wiley, New York
Effland RC, Helsley GC, Tegeler JJ (1982) Synthesis of 1,4-benzodiazepino[4,5-d][1,4]benzoxazepines. J Heterocycl Chem 19(3):537–539
Frisch MJ, Trucks GW, Schlegel HB et al (2004) Gaussian 03 Revision B. 05. Gaussian, Inc, Wallingford
Hallinan EA, Stapelfeld A, Savage MA et al (1994) 8-Chlorodibenz[B,F][1,4] oxazepine-10(11H)-carboxylic acid, 2-[3-[2-(furanylmethyl)thio]-1-oxopropyl]hydrazide (SC-51322)-a potent PGE(2) antagonist and analgesic. Bioorg Med Chem Lett 4(3):509–514
Hansch C, Leo A, Unger SH et al (1973) “Aromatic” substituent constants for structure- activity correlations. J Med Chem 16(11):1207–1216
Hansch C, Leo A, Taft RW (1991) A survey of Hammett substituent constants and resonance and field parameters. Chem Rev 91:165–195
HyperChem 7.2 for Windows (2002) Hypercube, Inc. USA
Kiska DI, Gilligan P, Pseudomonas H (1999) Manual of clinical microbiology. ASM, Washington, DC
Liegeois JFF, Rogister FA, Bruhwyler J et al (1994) Pyridobenzoxazepine and pyridobenzothiazepine derivatives a potential central-nervous-system agents—synthesis and neurochemical study. J Med Chem 37(4):519–525
Mishra JK, Samanta K, Jain M et al (2010) Amino acid based enantiomerically pure 3-substituted benzofused heterocycles: a new class of antithrombotic agents. Bioorg Med Chem Lett 20(1):244–247
Myers RH (1987) Classical and modern regression with application. PWS Publishers, Boston
Okada F, Torii Y, Saito H et al (1994) Antiemetic effects of serotonergic 5-HT1A-receptor agonists in Suncus murinus. Jpn J Pharmacol 2(64):109–114
Pekcec A, Unkruer B, Schlichtigeret J et al (2009) Targeting prostaglandin E-2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther 330(3):939–947
Schridhar DR, Sarma CR, Krishna RR et al (1979) Synthesis and anti-inflammatory activity of some 2,3-dihydro-1,4-benzoxazepin-5(4H)-one-7-acetic acid-esters. Indian J Chem Sect B 17B(2):155–157
Serrano-Wu MH, St Laurent DR, Chen YJ et al (2002) Sordarin oxazepine derivatives as potent antifungal agents. Bioorg Med Chem Lett 12(19):2757–2760
Sharma G, Park JY, Park MS (2008) Synthesis and anticonvulsant evaluation of 6-amino-1, 4-oxazepine-3,5-dione derivatives. Arch Pharm Res 31(7):838–842
Sleevi MC, Cale AD, Gero TW et al (1991) Optical isomers of rocastine and close analogs—synthesis and H1 antihistaminic activity of its enantiomers and their structural relationship to the classical antihistamines. J Med Chem 34(4):1314–1328
Smith L, Wong WC, Kiselyov AS et al (2006) Novel tricyclic azepine derivatives: biological evaluation of pyrimido[4,5-b]-1,4-benzoxazepines, thiazepines and diazepines as inhibitors of the epidermal growth factor receptor tyrosine kinase. Bioorg Med Chem Lett 16(19):5102–5106
Verma NK, Dempsey E, Conroy J et al (2008) A new microtubule-targeting compound PBOX-15 inhibits T-cell migration via post-translational modifications of tubulin. J Mol Med 86(4):457–469
Vogel AI (1972) Practical organic chemistry. Longman, London
Acknowledgment
The authors thank Kocaeli University Research Fund for financial support (Grant No. 2004/34).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agirbas, H., Kemal, B. & Budak, F. Synthesis and structure–antibacterial activity relationship studies of 4-substituted phenyl-4,5-dihydrobenzo[f][1,4]oxazepin-3(2H)-thiones. Med Chem Res 20, 1170–1180 (2011). https://doi.org/10.1007/s00044-010-9457-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9457-4