Abstract
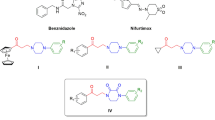
We describe herein an evaluation of the trypanocidal effect of eight piperamides (1–8) isolated from Piper tuberculatum bearing dihydropyridone, piperidine, and isobutyl moieties against epimastigote forms of Trypanosoma cruzi, the causative agent of Chagas’ disease. Based on such results, three hydrogenated and two hydrolyzed derivatives (10–14) were prepared and evaluated as well. The dihydropyridone amides (1–3) displayed higher anti-trypanosomal activity. The (Z)-piplartine (1) showed higher activity with a 50% inhibition concentration (IC50) value of 10.5 μM, almost four times more potent than the positive control, benznidazole (IC50 = 42.7 μM), and should be further evaluated as a suitable hit for the design of new antiprotozoal agents.


Similar content being viewed by others
References
Acharya AN, Saraswat D, Kaushik MP (2008) Antimalarial activity of Gomphostemma crinitum leaf extracts. Med Chem Res 17:530–540. doi:10.1007/s00044-008-9096-1
Araújo-Junior JX, Cunha EVL, Chaves MCO, Gray AI (1997) Piperdardine, a piperidine alkaloid from Piper tuberculatum. Phytochemistry 44:559–561. doi:10.1016/S0031-9422(96)00503-1
Batista-Júnior JM, Lopes AA, Ambrósio DL, Regasini LO, Kato MJ, Bolzani VS, Cicarelli RMB, Furlan M (2008) Natural chromenes and chromene derivatives as potential anti-trypanosomal agents. Biol Pharm Bull 31:538–540. doi:10.1248/bpb.31.538
Bernard CB, Krishnamurty HG, Chauret D, Durst T, Philogene BJR, Sanchez-Vindas P, Hasbun C, Poveda L, San Román L, Arnason JT (1995) Insecticidal defenses of Piperaceae from the neotropics. J Chem Ecol 21:801–814. doi:10.1007/BF02033462
Bezerra DP, Castro FO, Alves APNN, Pessoa C, Moraes MO, Silveira ER, Lima MAS, Elmiro FJM, Costa-Lotufo LV (2006) In vivo growth inhibition of sarcoma 180 by piperlonguminine, an alkaloid amide from the Piper species. Braz J Med Biol Res 39:801–807. doi:10.1590/S0100-879X2006000600014
Bialy L, Waldmann H (2003) Synthesis and biological evaluation of cytostatin analogues. Chem Commun (Camb) 15:1872–1873. doi:10.1039/b305308n
Bodiwala HS, Singh G, Singh R, Dey CS, Sharma SS, Bhutani KK, Singh IP (2007) Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J Nat Med 61:418–421. doi:10.1007/s11418-007-0159-2
Buck SB, Hardouin C, Ichikawa S, Soenen DR, Gauss CM, Hwang I, Swingle MR, Bonness KM, Honkanen RE, Boger DL (2003) Fundamental role of the fostriecin unsaturated lactone and implications for selective protein phosphatase inhibition. J Am Chem Soc 125:15694–15695. doi:10.1021/ja038672n
Castro JA, Mecca MM, Bartel LC (2006) Toxic side effects of drugs used to treat Chagas’ disease (American Trypanosomiasis). Hum Exp Toxicol 25:471–479. doi:10.1191/0960327106het653oa
Dias JCP, Silveira AC, Schofield CJ (2002) The impact of Chagas disease control in Latin America—a review. Mem Inst Oswaldo Cruz 97:603–612. doi:10.1590/S0074-02762002000500002
Duh CY, Wu YC, Wang SK (1990) Cytotoxic pyridone alkaloids from Piper aborescens. Phytochemistry 29:2689–2691. doi:10.1016/0031-9422(90)85215-2
Freire-de-Lima L, Ribeiro TS, Rocha GM, Brandão BA, Romeiro A, Mendonça-Previato L, Previato JO, Lima MEF, Carvalho TMU, Heise N (2008) The toxic effects of piperine against Trypanosoma cruzi: ultrastructural alterations and reversible blockage of cytokinesis in epimastigote forms. Parasitol Res 102:1059–1067. doi:10.1007/s00436-008-0876-9
Isao K (1984) Molluscicidal and insecticidal activities of isobutylamides isolated from Fagara macrophylla. Experientia 40:340–341. doi:10.1007/BF01952540
Joshi BS, Kamat VN, Saksena AK (1968) On the synthesis of piplartine and a synthesis of dihydropiplartine. Tetrahedron Lett 20:2395–2400. doi:10.1016/S0040-4039(00)76140-5
Kapil A (1993) Piperine: a potent inhibitor of Leishmania donovani promastigotes in vitro. Planta Med 59:474. doi:10.1055/s-2006-959737
Lebedev AV, Lebedeva AB, Sheludyakov VD, Kovaleva EA, Ustinova OL, Kozhevnikov IB (2005) Synthesis of 3-substituted arylpyrazole-4-carboxylic acids. Russ J Gen Chem 75:1113–1124. doi:10.1007/s11176-005-0377-9
Lopes AA, López SN, Regasini LO, Batista-Júnior JM, Ambrósio DL, Kato MJ, Bolzani VS, Cicarelli RM, Furlan M (2008) In vitro activity of compounds isolated from Piper crassinervium against Trypanosoma cruzi. Nat Prod Res 22:1040–1046. doi:10.1080/14786410802243271
Madhusudhan P, Vandana KL (2001) Tetrahydropiperine, the first natural aryl pentanamide from Piper longum. Biochem Syst Ecol 29:537–538. doi:10.1016/S0305-1978(00)00083-1
Maxwell A, Rampersad D (1991) A new dihydropiplartine and piplartine dimmer from Piper rugosum. J Nat Prod 54:1150–1152. doi:10.1021/np50076a044
Mishra S, Narain U, Mishra R, Misra K (2005) Design, development and synthesis of mixed bioconjugates of piperic acid–glycine, curcumin–glycine/alanine and curcumin–glycine–piperic acid and their antibacterial and antifungal properties. Bioorg Med Chem 13:1477–1486. doi:10.1016/j.bmc.2004.12.057
Molina-Torres J, Salazar-Cabrera CJ, Armenta-Salinas C, Ramírez-Chávez E (2004) Fungistatic and bacteriostatic activities of alkamides from Heliopsis longipes roots: affinin and reduced amides. J Agric Food Chem 52:4700–4704. doi:10.1021/jf034374y
Muelas-Serrano S, Nogal-Ruiz JJ, Gómez-Barrio A (2000) Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol Res 86:999–1002. doi:10.1007/PL00008532
Navickiene HMD, Alécio AC, Kato MJ, Bolzani VS, Young MCM, Cavalheiro AJ, Furlan M (2000) Antifungal amides from Piper hispidum and Piper tuberculatum. Phytochemistry 55:621–626. doi:10.1016/S0031-9422(00)00226-0
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477. doi:10.1021/np068054v
Nozoe T, Tanimoto K, Takemitsu T, Kitamura T, Harada T, Osawa T, Takayasu O (2001) Non-solvent hydrogenation of solid alkenes and alkynes with supported palladium catalysts. Solid State Ion 141:695–700. doi:10.1016/S0167-2738(01)00781-0
Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A, Tyagi OD, Prasad AK, Wengel J, Olsen CE, Boll PM (1997) Phytochemistry of the genus Piper. Phytochemistry 46:597–673. doi:10.1016/S0031-9422(97)00328-2
Raay B, Medda S, Mukhopadhyay S, Basu MK (1999) Targeting of piperine intercalated in mannose-coated liposomes in experimental leishmaniasis. Indian J Biochem Biophys 36:248–251
Reddy SV, Srinivas PV, Praveen B, Kishore H, Raju BC, Murthy US, Rao JM (2004) Antibacterial constituents from the berries of Piper nigrum. Phytomedicine 11:697–700. doi:10.1016/j.phymed.2003.04.004
Ribeiro TS, Freire-de-Lima L, Previato JO, Mendonça-Previato L, Heise N, de Lima MEF (2004) Toxic effects of natural piperine and its derivatives on epimastigotes and amastigotes of Trypanosoma cruzi. Bioorg Med Chem Lett 14:3555–3558. doi:10.1016/j.bmcl.2004.04.019
Rosario SL, Silva AJR, Parente JP (1996) Alkamides from Cissampelos glaberrima. Planta Med 62:376–377. doi:10.1055/s-2006-957913
Rukachaisirikul T, Siriwattanakit P, Sukcharoenphol K, Wongvein C, Ruttanaweang P, Wongwattanavuch P, Suksamrarn A (2004) Chemical constituents and bioactivity of Piper sarmentosum. J Ethnopharmacol 93:173–176. doi:10.1016/j.jep.2004.01.022
Settimj G, Simone L, Giudice MR (1976) A new gas chromatographic method for the estimation of reserpine and rescinnamine. J Chromatogr A 116:263–270. doi:10.1016/S0021-9673(00)89897-0
Silva RV, Navickiene HMD, Kato MJ, Bolzani VS, Méda CI, Young MCM, Furlan M (2002) Antifungal amides from Piper arboreum and Piper tuberculatum. Phytochemistry 59:521–527. doi:10.1016/S0031-9422(01)00431-9
Singh V, Tripathi CKM, Bihari V (2008) Production, optimization and purification of an antifungal compound from Streptomyces capoamus MTCC 8123. Med Chem Res 17:94–102. doi:10.1007/s00044-007-9040-9
Tripathi CKM, Praveen V, Singh V, Bihari V (2004) Production of antibacterial and antifungal metabolites by Streptomyces violaceusniger and media optimization studies for the maximum metabolite production. Med Chem Res 13:790–799. doi:10.1007/s00044-004-0118-3
Viswanathan MB, Ramesh N, Ahilan A, Lakshmanaperumalsamy P (2004) Phytochemical constituents and antimicrobial activity from the stems of Jatropha maheshwarii. Med Chem Res 13:361–368. doi:10.1007/s00044-004-0041-7
Wang T, Zhang Y, Pei Y (2007) Two novel trichothecenes from Myrothecium roridum. Med Chem Res 16:155–161. doi:10.1007/s00044-007-9012-0
Acknowledgements
This work was funded by grants provided by FAPESP (03/11524-9) and also supported within the BIOTA/FAPESP—Biodiversity Virtual Institute Program (www.biota.org.br). M.F., M.J.K., and V.S.B. are grateful to CNPq for research fellowships. F.C. (07/56140-4) and L.O.R. (03/00886-7) wish to thank FAPESP for providing scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cotinguiba, F., Regasini, L.O., da Silva Bolzani, V. et al. Piperamides and their derivatives as potential anti-trypanosomal agents. Med Chem Res 18, 703–711 (2009). https://doi.org/10.1007/s00044-008-9161-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-008-9161-9