Abstract
Synapsin I (SynI) is a synaptic vesicle (SV)-associated phosphoprotein that modulates neurotransmission by controlling SV trafficking. The SynI C-domain contains a highly conserved ATP binding site mediating SynI oligomerization and SV clustering and an adjacent main Ca2+ binding site, whose physiological role is unexplored. Molecular dynamics simulations revealed that the E373K point mutation irreversibly deletes Ca2+ binding to SynI, still allowing ATP binding, but inducing a destabilization of the SynI oligomerization interface. Here, we analyzed the effects of this mutation on neurotransmitter release and short-term plasticity in excitatory and inhibitory synapses from primary hippocampal neurons. Patch-clamp recordings showed an increase in the frequency of miniature excitatory postsynaptic currents (EPSCs) that was totally occluded by exogenous Ca2+ chelators and associated with a constitutive increase in resting terminal Ca2+ concentrations. Evoked EPSC amplitude was also reduced, due to a decreased readily releasable pool (RRP) size. Moreover, in both excitatory and inhibitory synapses, we observed a marked impaired recovery from synaptic depression, associated with impaired RRP refilling and depletion of the recycling pool of SVs. Our study identifies SynI as a novel Ca2+ buffer in excitatory terminals. Blocking Ca2+ binding to SynI results in higher constitutive Ca2+ levels that increase the probability of spontaneous release and disperse SVs. This causes a decreased size of the RRP and an impaired recovery from depression due to the failure of SV reclustering after sustained high-frequency stimulation. The results indicate a physiological role of Ca2+ binding to SynI in the regulation of SV clustering and trafficking in nerve terminals.
Similar content being viewed by others
Introduction
A cohort of proteins operates synaptic vesicle (SV) exocytosis at nerve terminals and fine-tune the efficiency of neurotransmitter release. Three main functional SV pools exist: a readily releasable pool (RRP) of SVs docked/primed at the active zone, a recycling pool (RecP) of SVs that refill the RRP depleted upon the activity, and a resting pool (RestP) of SVs that cannot be released by electrical activity and represent a functional reserve [2, 18, 68]. The Synapsins (SynI/II/III) constitute an abundant family of SV-associated phosphoproteins that play important roles in synaptogenesis and in the regulation of clustering and mobilization of SVs in presynaptic terminals [11, 20, 28, 50, 56]. SynI and SynII, the major isoforms in mature neurons, have both redundant and distinct functions in synaptic transmission. While both contribute to the formation and maintenance of the RecP by promoting SV clustering [7, 8, 40, 58], they have complementary roles in synchronous and asynchronous release [29, 53, 54]. Moreover, SynI is at the convergence of multiple signaling pathways and is phosphorylated during neural activity [43]. Phosphorylation of SynI by Ca2+/calmodulin (CaM)-dependent and cAMP-dependent kinases inactivates SV clustering by SynI, releasing SVs toward the RRP [12, 13], while phosphorylation/dephosphorylation of SynI by Cdk5 and calcineurin, respectively, regulates the transitions of SVs from the RestP to the RecP [83]. All Syn isoforms display a highly conserved ATP binding site in the central C-domain [23]. The binding of ATP was initially reported to be differentially regulated by Ca2+ ions in the three Syn isoforms, being stimulated, unaffected, or inhibited in Syns I, II, and III, respectively [39] and proposed to increase SynI oligomerization [10]. Afterward, molecular dynamics (MD) simulations revealed that ATP binding is mediated by a conformational transition of a flexible loop (multifunctional loop, MFL) that opens to render the ATP site accessible to the ligand [62]. This conformational change is not affected by the absence of Ca2+ and increases the association of SynI with SVs and SV clustering [62]. These findings raise questions on the functional role of Ca2+ binding to SynI. In the SynI C- domain crystal structure, Ca2+ is coordinated by the pyrophosphate moiety of ATP and two glutamate residues (Glu373 and Glu386; [23]) and its presence shifts the ATP-induced SynI dimerization to tetramerization [62]. Since Ca2+ recruitment is coordinated by Glu373 and is abolished by Glu-Lys substitution without affecting the conformation of the ATP binding pocket [39, 62], we dissected the functional role of Ca2+ binding to SynI by re-introducing either wild-type SynI (SynIWT) or the SynIE373K mutant in SynI knockout (KO) neurons. MD simulations revealed that ATP and Ca2+ binding to SynI can occur independently, although they facilitate each other, and that the E373K mutation affects the oligomerization interface of SynI. We found that the deletion of Ca2+ binding to SynI increases the resting levels of intraterminal Ca2+ and the probability of spontaneous SV exocytosis in excitatory neurons. Moreover, expression of the SynIE373K mutant decreases SV density and size of the RRP, resulting in a smaller amplitude of evoked excitatory currents. Such effects are not present in inhibitory synapses, indicating that, the Ca2+-buffering activity of SynI is dispensable in inhibitory neurons. In both excitatory and inhibitory synapses, however, deletion of Ca2+ binding to SynI leads to SV depletion and markedly impairs recovery from synaptic depression. The results indicate SynI as a new Ca2+-buffer for excitatory neurons and indicate that Ca2+ binding to SynI has important roles in regulating SV trafficking across functional SV pools.
Materials and methods
MD simulations
MD simulations of solvated SynIWT and SynIE373K C-domains were previously analyzed [62] using the program NAMD [66] and the CHARMM27 force field with the CMAP correction for protein backbone energetics [51]. Simulations lasted 150 ns and were performed at 300 K. All parameters and set-ups of the simulations are described in Orlando et al. [62]. The various simulated systems were built by immersing the protein monomer in a box of TIP3P model water molecules [42] and counter-ions to neutralize the total charge, for a total of 63,000 atoms in all cases. ATP or Ca2+ was removed where necessary by deleting the corresponding atoms and the mutations were introduced by replacing the side chains of the involved residues. In all simulations, periodic boundary conditions were used to replicate the system and remove box surface effects [30]. Short-range nonbonded interactions were cutoff at 12 Å, whereas long-range electrostatic interactions were computed using the particle mesh Ewald method [19]. Chemical bonds connecting hydrogen atoms to heavy atoms were kept fixed using SHAKE [71]. The integration time step was 1 fs to ensure the stability of the molecular dynamics algorithm. A proper size for the simulation box corresponding to a pressure of 1 atm was obtained by simulating the system in the constant pressure and temperature ensemble using the Nosé́-Hoover Langevin piston method for 0.5 ns [52]. The simulation ensemble was then switched to a constant volume and temperature (NVT) ensemble for the rest of the simulation by keeping the temperature stationary at 300 K using Langevin dynamics. Protein atomic coordinates were obtained from the crystal structure of rat SynI C-domain complexed with ATP and Ca2+ (PDB code 1pk8; [10]. A 200 ns-long simulation of the wild-type (WT) SynI C-domain without ATP and with one Ca2+ at the binding site was performed here with the same parameters and set-ups of the WT and E373K trajectories. Calcium ion pulling simulations were performed starting from conformations extracted from the WT ATP/no-ATP Ca2+ trajectories and using a harmonic restraint on the distance between the ion and the Ca2+ atoms of residues V372, E373, E386, and V387, with a target linearly increasing from the starting value (about 7 Å) to 40 Å in 10 ns. Five simulations were performed for ATP/Ca2+ SynI and five for no-ATP/Ca2+ SynI, always using the same force constant k = 0.8 kcal/(mol*Å2).
Experimental animals
Syn I KO mice were generated by homologous recombination and extensively backcrossed on the C57BL/6J background for > 10 generations [48]. Mice were housed under constant temperature (22 ± 1 °C) and humidity (50%) conditions with a 12 h light/dark cycle and were provided with food and water ad libitum. All experiments were carried out in accordance with the guidelines established by the European Community Council (Directive 2010/63/EU of September 22, 2010) and were approved by the Italian Ministry of Health (authorization no. 605/2021-PR).
Low-density and autaptic cultures of SynI KO hippocampal neurons
Pregnant females were sacrificed by inhalation of CO2, and embryonic day 17–18 (E17–18) embryos were removed immediately by cesarean section. Hippocampi were digested in 0.125% trypsin for 15 min, mechanically dissociated with a fire-polished Pasteur pipette, and finally plated at low density (40 cells/mm2). Autaptic neurons were prepared as described previously [6]. Briefly, dissociated neurons were plated at very low density (20 cells/mm2) on microdots (40–400 µm in diameter) obtained by spraying a mixture of poly-l-lysine (0.1 mg/mL) on dishes which had been pretreated with 0.15% agarose. All hippocampal cultures were maintained in a culture medium consisting of Neurobasal, 2% B-27, 1% Glutamax, and 1% penicillin/streptomycin in a humidified 5% CO2 atmosphere at 37 °C.
Virus production and neuron transduction
Sequences containing mCherry-tagged SynWT and SynIE373K were cloned into pLenti6.2/V5-Dest plasmids (Invitrogen). As a control for viral transduction, an empty EGFP-expressing lentiviral construct pCCL-sin-PPT-prom-EGFP-WPRE was used (referred to in the manuscript as SynIKO). The production of VSV-pseudo-typed third-generation lentiviruses was performed as previously described [21]. Viral titers of about 1.0 × 109 TU/mL were obtained for all vectors. For all the experiments, hippocampal neurons were infected at 6–7 DIV at 10-multiplicity of infection. After 24 h from the infection, half of the medium was replaced with fresh medium. The transduction efficiency was assessed by counting the number of mCherry-positive cells with respect to the number of Hoechst-positive nuclei using ImageJ.
Patch-clamp recordings from dissociated and autaptic cultured hippocampal neurons
Cultured hippocampal neurons obtained from Syn I KO mice embryos were recorded at 12–15 DIV. Patch pipettes, prepared from thin borosilicate glass, were pulled and fire-polished to a final resistance of 4–5 MΩ when filled with a standard internal solution. Voltage-clamp recordings were performed at a holding potential of − 70 mV and acquired at 10–20 kHz sample frequency. All experiments were performed at room temperature (22–24 °C). Data acquisition was performed using the PatchMaster program (HEKA Elektronic). For all the experiments, cells were maintained in an extracellular standard solution (Tyrode) containing (in mM): 140 NaCl, 2 CaCl2, 1 MgCl2, 4 KCl, 10 glucose, and 10 HEPES (pH 7.3 with NaOH). The internal solution (K-gluconate) was composed of (in mM) 126 K gluconate, 4 NaCl, 1 MgSO4, 0.02 CaCl2, 0.1 BAPTA, 15 glucose, 5 HEPES, 3 ATP, and 0.1 GTP (pH 7.3 with KOH). Evoked excitatory postsynaptic currents (eEPSCs) were recorded from autaptic neurons, maintained in a Tyrode external solution supplemented with: d-(–)-2-amino-5-phospho-nopentanoic acid (D-AP5; 50 μM) and bicuculline (BIC, 30 μM) to block N-methyl-d-aspartate (NMDA) and GABAA receptors, respectively. To selectively block AMPA receptor desensitization experiments were run in the presence of cyclothiazide (CTZ; 60 µM). The autaptic neurons under study were voltage clamped at a holding potential (Vh) of −70 mV. Unclamped action potentials (APs) evoking EPSCs were activated by a brief depolarization of the cell body to + 40 mV for 0.5 ms. Evoked inhibitory postsynaptic currents (eIPSCs) were recorded in low-density hippocampal neurons perfused with Tyrode solution supplemented with D-AP5 (50 μM), CNQX (10 μM), and CGP58845 (5 μM) to block NMDA, non-NMDA, and GABAB receptors, respectively. eIPSCs were evoked by extracellular electrical stimulation, positioning the stimulating glass microelectrode, filled with the external Tyrode solution in the proximity of a putative presynaptic neuron and applying increasing current to generate a postsynaptic response in the postsynaptic voltage-clamped neuron. Extracellular stimulation was performed by administering test pulses of 0.5 ms, generally ranging between 10 and 60 pA. In particular, the stimulation intensity was progressively increased to evoke minimal eIPSCs and such value was further augmented by 50%, in order to avoid “failures” during tetanic high frequency stimulation. The size of the readily releasable pool of synchronous release (RRPsyn) and the probability release (Pr) was calculated using the cumulative amplitude analysis during 2–2.5 s tetanic stimulation at 40 Hz [73]. Data points in the linear range of the curves (between 0.5 and 1 s) were fitted by linear regression and retrogradely extrapolated to time 0. The intercept with the Y-axis gave the RRP and the ratio between the amplitude of the first ePSC (I1) and RRP yielded the Pr. To study the response to paired-pulse protocols, we applied two consecutive stimuli at increasing interpulse intervals (20–1000 ms). The paired-pulse ratio (PPR; I2/I1) was calculated by dividing the amplitude of the second response (I2) by the amplitude of the first one (I1). Miniature postsynaptic currents (mPSCs) were recorded in the voltage-clamp configuration in the presence of tetrodotoxin (TTX; 300 nM) in the extracellular solution to block the generation and propagation of spontaneous action potentials. The amplitude and frequency of mPSCs were calculated using a peak detector function using appropriate threshold amplitude and area. The frequency, amplitude, and kinetics of miniature PSCs were analyzed using the MiniAnalysis program (Synaptosoft, Inc.) and the Prism software (GraphPad Software, Inc.). All reagents were obtained by Tocris, otherwise specified.
Immunofluorescence
Primary hippocampal neurons were washed once in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature. Cells were blocked with blocking buffer [5% goat serum (Vector Labs), 0.5% Triton X-100] in PBS for 1 h at room temperature. Samples were sequentially incubated with primary antibodies overnight at 4 °C, followed by incubation with fluorochrome-conjugated secondary antibodies (Invitrogen) for 2 h at room temperature. After several washes in PBS, coverslips were mounted using Mowiol (Invitrogen). For quantification of the number of inhibitory and excitatory synapses, cultured neurons were stained for vGAT (Synaptic Systems; 1:500 dilution) and vGLUT1 (Synaptic Systems; 1:200 dilution). For the analysis of synaptic density, samples were visualized using a 63 × objective in a fluorescence microscope (Leica). Several z-stacks (18–35 for each image; step size, 0.35 μm) were acquired. Image analysis was performed with ImageJ by superimposing stacks of each color channel. The density of puncta was calculated as the number of vGLUT1- or vGAT-positive puncta per 30 μm of proximal dendrite length. Manders’ colocalization coefficient was calculated using the ImageJ JACoP plugin and expressed as the percentage of mCherry-SynI signal overlapping with either vGAT or vGLUT1 immunoreactivity.
Live imaging experiments
Presynaptic Ca2+ imaging with SynGCaMP6f
Imaging was performed in hippocampal primary cultures at room temperature (22–24 °C) in Tyrode solution. Cultures were coinfected with SynGCaMP6f (#40755 Addgene; virus provided by the Viral Core Facility of Charité—Universitätsmedizin Berlin) and either SynIWT or SynIE373K viral vectors in a 1:1 ratio 6–7 days before the experiments and measured at 14–15 DIV. Coverslips were mounted in a Chamlide stimulation chamber (Live Cell Instrument, Seoul, Korea) on the stage of an Olympus IX-71 inverted microscope fitted with a UplanSapo 60X1.35 NA oil-immersion objective (Olympus) and an Orca-ER IEEE1394 CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan, resolution 1344 × 1024). A 460–495 nm excitation/510–550 nm emission filter set was used for SynGCaMP6f. Time lapses were acquired at binning 2 with a 1 Hz frequency (200-ms exposure), for the whole experimental time. APs were evoked by passing 10 V, 1 ms current pulses from a custom-made stimulation box via platinum electrodes at increasing frequencies (0.5–100 Hz) for 2 s. Stimulation, image acquisition, and shuttering were all under the coordinated control of the WinWCP software. Images were analyzed in ImageJ and analyzed with Prism software (GraphPad Software, Inc.). Regions of interest (ROIs) were positioned on all boutons that colocalized with mCherry-positive puncta and fluorescent signals were normalized to the background. The experimental setting was designed as follows: cells were imaged for 15 s of baseline, after which a 2-s train of APs at increasing frequencies was administered and fluorescence was recorded until 30 s. For the basal recordings, the fluorescent SynGCaMP6f signals, recorded for 15 s under non-stimulated conditions, were normalized to the local background signal. For activity-dependent recordings, ROIs were positioned on all boutons responding to 20 APs for SynGCaMP6f. Evoked signals were quantified by normalizing to the local background noise and the non-stimulated (basal) signal.
Calcium imaging with Fura-2 AM
Primary hippocampal neurons (7 DIV) were transduced with either SynIWT or SynIE373K fused to mCherry and imaging was performed at 14/15 DIV. Cells were loaded with 1 μg/mL cell-permeable Fura-2 AM (#F1221, ThermoFisher) in the culture medium and maintained for 30 min in the incubator. Cells were then washed with culture medium and incubated for 30 min to allow hydrolysis of the esterified groups. Coverslips with cells were mounted in the imaging chamber and loaded with 0.5 mL of recording buffer (150 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES pH 7.4). Fura-2 loaded cultures were observed with an IX-81 motorized inverted epifluorescence microscope (Olympus, Tokyo, Japan) using a UplanSapo 63 × 1.35 NA oil-immersion objective (Olympus). Regions of interest (ROIs) were positioned on all boutons that colocalized with mCherry-positive puncta. Samples were excited at 340 and 380 nm by an MT20 Hg–Xe lamp (Olympus). Excitation light was separated from the emitted light using a 395 nm dichroic mirror. Images of fluorescence emission > 510 nm were acquired continuously for 300 s using a Hamamatsu Orca-ER IEEE1394 CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan). The camera operated on 2 × 2-pixel binning mode, and the imaging system was controlled by an integrating imaging software package (Cell∧R; Olympus). Ca2+ concentration was calculated as in Grynkiewicz et al. [36], using the following equation:
where R is the measured 340/380 nm ratio; Rmin and Rmax are the ratios in the absence of Ca2+ or when Fura-2 is saturated by Ca2+ and were determined by incubating cells in 0 Ca2+/5 mM EGTA or treating cells with 1 μM ionomycin in recording buffer containing increasing concentrations of Ca2+ (from 1 nM to 10 mM). F380max and F380min are the fluorescence intensities of 380 nm excitation at 0 Ca2+ and Ca2+ saturation, respectively. The calibration was performed for each cell preparation by loading cells with Fura-2, then measuring the 340/380 nm ratios in Ca2+ buffer mixtures that covered the [Ca2+] range of interest (r2 = 0.883 ± 0.048; mean ± SEM). To obtain the calibration curve, values were plotted and the x-intercept representing the log of the Kd in the Grynkiewicz equation was calculated by linear regression. Recorded images were analyzed by ImageJ and Prism6 softwares.
Imaging of SV cycling with synaptopHluorin (sypHy)
Primary hippocampal neurons (10 DIV) were transduced with sypHy-GFP and either SynIWT or SynIE373K fused to mCherry. At 18–21 DIV live imaging recordings coupled with electric field stimulations were performed in Quick Exchange Platform (Warner Instruments) by applying 1-ms current pulses through platinum–iridium electrodes using an AM2100 stimulator (AM-Systems). Blocking of glutamate receptors in neuronal cultures was achieved by adding CNQX and APV (10 and 50 µM respectively) to the Tyrode solution. The experimental setting was designed as follows: (i) cells were imaged for 10 s (baseline); (ii) a train of 20 APs @ 100 Hz was applied and fluorescence recorded for further 90 s, a protocol repeated three times; (iii) at the end, a larger stimulus (600 AP @ 20 Hz) was applied and fluorescence recorded for 120 s; (iv) after 80 s of resting to avoid bleaching, another round of three 20 APs @ 100 Hz stimulations was applied; (v) at the end of the experimental protocol, cells were perfused with 50 mM NH4Cl-Tyrode to calculate the total fluorescence at responsive synaptic terminals (Fmax). Fluorescence intensity at synaptic boutons was measured by manually applying circular regions of interests (ROIs) of 1.7 µm diameter at the center of each responsive synapse expressing either SynIWT or SynIE373K. The increase in the fluorescence signal (ΔF) upon the 20 APs @ 100 Hz stimulation normalized to the basal fluorescence (F0, calculated as the average of the first 5 s before the stimulus application after background subtraction) is a measure of the RRP (ΔF/F0). The endocytosis rate (τ Endo) was calculated by fitting the post-stimulus decay with the single-exponential function ΔF/F0 (t) = (ΔF/F0)∞ (t = ∞) + ΔF/F0 (t = 0) ⋅ e−t/τ. The normalized means of three peaks before (train 1) and after (train 2) the long-lasting stimulation were evaluated to calculate recovery. Three independent cell preparations were performed for each experimental condition and an average of 32 synaptic boutons per field were analyzed.
Electron microscopy
Cultured hippocampal neurons derived from SynI KO mice were infected at 7 DIV with either SynIWT or SynIE373K and fixed at 14 DIV with 1.2% glutaraldehyde in 66 mM sodium cacodylate buffer, post-fixed in 1% OsO4, 1.5% K4Fe(CN)6, 0.1 M sodium cacodylate, en bloc stained with 1% uranyl acetate, dehydrated and flat-embedded in epoxy resin (Epon 812, TAAB). After baking for 48 h, the glass coverslips were removed from the Epon block by thermal shock and neurons were identified by means of a stereomicroscope. Embedded neurons were excised from the block and mounted on a cured Epon block for sectioning using an EM UC6 ultramicrotome (Leica Microsystems). Ultrathin sections (60–70 nm thick) were collected on mesh copper grids (Electron Microscopy Sciences) and observed with a JEM-1011 electron microscope (Jeol) operating at 100 kV using an ORIUS SC1000 CCD camera (Gatan, Pleasanton). For each experimental condition, at least 30 images of synapses were acquired at 10,000 × magnification (sampled area per experimental condition: 36 μm2). SV density and docked SV linear density were determined using ImageJ software. The analysis of synaptic ultrastructure at the end of the train stimulation protocol (30 s at 20 Hz) or after recovery (120 s at 0.1 Hz) was performed by quickly fixing the samples with glutaraldehyde at 37 °C. Under these conditions, we estimated a complete fixation of synapses within 1–3 s from fixative addition [47].
Statistical analysis
Data are expressed as box plots or means ± sem (in case of normal distribution) for n = sample size. Normal distribution was assessed using D’Agostino and Pearson’s normality test. The box plot elements are the following: center line, median (Q2); cross symbol, mean; box limits, 25th (Q1)–75th (Q3) percentiles; whisker length is determined by the outermost data points within threefold the interquartile range (Q3–Q1). To compare two sample groups, the Student’s t-test and the Mann–Whitney U-test were used for normally and non-normally distributed data, respectively. To compare more than two normally distributed sample groups, one- or two-way ANOVA followed by multiple comparison tests (Bonferroni’s, Tukey’s, or Dunnett’s tests) were used. To compare more than two sample groups that were not normally distributed, Kruskal–Wallis’s ANOVA followed by the Dunn’s multiple comparison test was used. The significance level was preset to p < 0.05 for all tests. Statistical analysis was carried out using OriginPro-9 (OriginLab Corp.) and Prism (GraphPad Software, Inc.).
Results
The presence of ATP strengthens binding of Ca2+ to the main ATP/Ca2+ binding site in the C-domain of SynI
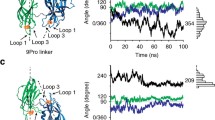
We have previously shown that ATP can bind to SynI also in the absence of Ca2+, questioning the classical view that Ca2+ binding solely serves to regulate ATP binding [62]. Here, we first addressed the possibility that Ca2+ and ATP bind independently of each other to the SynI C-domain and that Ca2+ binding plays a further functional role in addition to enhancing ATP binding. Figure 1A shows the structure of the SynI C-domain together with the details of the ATP binding site in SynIWT and in the simulated model of the SynIE373K mutant (Fig. 1B, top and bottom panels, respectively). In the SynIWT protein, the binding pocket is covered by the MFL that contacts ATP and prevents water access. The MFL, together with the phosphate binding loop (PBL) and loop 6 (Fig. 1A), also contains residues that are relevant for the formation of SynI oligomers. In the SynIWT structure, the Ca2+ ion is coordinated by all three ATP phosphate groups, according to the second most observed geometry in experimentally determined structures of proteins containing ATP and a divalent cation [49]. Oxygen atoms from glutamic acid residues E373 and E386 also coordinate Ca2+ (Fig. 1B, top panel).
Interdependence of the ATP and Ca2+ binding sites in the SynI C-domain. A Pictorial representation of the SynI C-domain. The Ca2+ atom is represented as a yellow sphere, while the ATP molecule and residues E369 and E373 as sticks. The MFL, PBL, and loop 6 are indicated and colored in blue, red, and orange, respectively. B Enlarged view of the ATP binding site in WT (top) and E373K (bottom) SynI. C Left: Number of Ca2+-coordinating oxygen atoms (within a sphere of 3 Å) during the simulation of WT SynI without ATP, from water (black dots), E373 and E386 (red dots), and total (blue dots). Middle: Snapshot extracted from the simulation showing the Ca2+ atom (yellow sphere) coordinated by the oxygen atoms from E373, E386, and four water molecules. Right: Distance of Ca2+ from the main binding site in pulling simulations without ATP (black, purple, orange, brown, and green lines) and with ATP (blue line, representative trajectory); the red line shows the target distance of the ion from the starting site, linearly increasing during the simulation time. D Histograms of RMSD values for the PBL (left) and the loop 6 (right) in WT and E373K SynI from the corresponding simulations
In our model of the SynIE373K mutant, Ca2+ is absent and the ATP molecule interacts with the substituting lysine (Fig. 1B; bottom panel). We next asked whether a Ca2+ ion can bind also in the absence of ATP to the main ATP-Ca2+ binding site of the SynIWT C-domain revealed in the crystal structure. Thus, we simulated the SynIWT monomer after removing the ATP molecule and leaving the Ca2+ ion at the site. We observed that, along the entire simulated trajectory (200 ns), the ion remained stably tethered at the same site, tightly coordinated by E373 and E386 and an average number of four water molecules (Fig. 1C; left and middle panels). Indeed, when ATP is not present, although the MFL loop remains closed over the binding pocket as in SynIWT [62], more water molecules can access the site with respect to the WT trajectory, thus providing coordinating oxygen atoms to the Ca2+ ion.
We then investigated how ATP affects the strength of Ca2+ binding to SynI. To this purpose, we ran several simulations in which we pulled the Ca2+ ion away from the main site by applying an external force to linearly increase the distance (we applied a harmonic restraint, or a “spring”, on the distance with a linearly increasing target; see “Materials and methods” section). Five simulations were carried out with ATP bound, and five without ATP bound, keeping the force constant. We observed that, while in the SynI-noATP simulations Ca2+ moved away from the binding site (Fig. 1C; right panel), in the SynI-ATP simulations the Ca2+-ATP complex remained tightly tethered at the main binding site (Fig. 1C; blue line in the right panel). Thus, being in complex with ATP increases the strength of Ca2+ interaction with SynI, indicating that Ca2+ and ATP can reciprocally enhance the binding of the respective partner to the binding site [62]. We previously demonstrated that mutations of the ATP binding site in SynI affected its oligomerization dynamics. To examine the possible consequences of E373K mutation on the formation of SynI tetramers, we analyzed the behavior of the tetramer interface residues during the MD simulations of SynIWT and SynIE373K presented in Orlando et al. [62]. We observed that, although ATP remains at the binding site (Fig. 1B, bottom panel) and the MFL is closed over the pocket, the mutation enhances conformational disorder in the PBL (Fig. 1D, left panel) and in the loop 6 (Fig. 1D, right panel). Because these segments contain residues that establish direct contacts between monomers to form the tetramer, their distortion results in the perturbation of the interactions that stabilize the oligomers and are involved in SV clustering.
Expression of SynIE373K in a SynI KO background increases the frequency of miniature postsynaptic currents only in excitatory synapses
Based on the suggestions by MD simulations that Ca2+ binds with high affinity at the ATP binding site and affects the tetramerization surface, we proceeded to physiologically test the SynIE373K mutant that abolishes the main Ca2+ binding, without affecting the binding of ATP [62]. To address the functional role of Ca2+ binding to SynI using lentiviral vectors and low-density primary hippocampal neurons from SynI KO mice, we expressed: (i) a reporter-containing empty vector for the control KO group (SynIKO; (ii wild type mCherry-SynI (SynIWT); and (iii) mCherry-SynI mutant in the Ca2+ binding site (SynIE373K). Analysis of the fluorescent reporters showed that the vast majority of the neurons (SynIKO: 96.41 ± 0.34; SynIWT: 95.13 ± 0.25; SynIE373K: 94.74 ± 0.27) were transduced with comparable expression levels. We first investigated whether the expression of SynIE373K could alter the physiological properties of excitatory (Fig. 2A, B) and inhibitory (Fig. 2C, D) miniature postsynaptic currents (mEPSCs and mIPSCs, respectively) as compared to SynIWT and SynIKO. The expression of the SynIE373K mutant markedly increased the frequency of mEPSCs with respect to SynKO neurons or neurons rescued with SynWT, while the amplitude was not affected (Fig. 2A; lower panels).
Expression of the SynIE373K mutant selectively increases mEPSC frequency. A, B mEPSCs. Top panels: Representative traces recorded at −70 mV in 14 DIV SynIKO hippocampal neurons expressing empty vector (orange), SynIWT (black), or SynIE373K (red) (A) and single events in an expanded time scale (B). Bottom panels: Box plots of mEPSC frequency and amplitude (A) and rise and decay times obtained by fitting individual currents (B). C, D mIPSCs. Top panels: Representative traces recorded at −70 mV in 14 DIV SynIKO hippocampal neurons expressing empty vector (orange), SynIWT (black), or SynIE373K (red) (C) and single events in an expanded time scale (D). Bottom panels: Box plots of mEPSC frequency and amplitude (C) and rise and decay times obtained by fitting individual currents (D). Excitatory synapses: n = 19, 20, 20; Inhibitory synapses: n = 14, 17, 16; for SynIKO, SynIWT, and SynIE373K, respectively from n = 3 independent neuronal preparations. *p < 0.05; **p < 0.01; Kruskal–Wallis/Dunn’s tests
On the contrary, no changes in the frequency and amplitude of mIPSCs were observed across the three genotypes (Fig. 2C; lower panels). The analysis of the rise and decay times revealed no significant changes in the kinetics of both mEPSCs (Fig. 2B) and mIPSCs (Fig. 2D) across the three genotypes. The results uncover a strong and selective effect of the SynI Ca2+ mutant on the mEPSC frequency with preservation of the quantal size, thus excluding the presence of secondary postsynaptic effects. The data also confirm that the Syn1deletion per se does not significantly affect the properties of mPSCs, as previously reported [14].
SynIE373K does not influence the density of excitatory and inhibitory synapses
An increased frequency of miniature synaptic events is normally attributable to either an increased number of synapses or an increased probability of spontaneous SV fusion. To test the first possibility, we investigated whether the expression of SynI variants could differentially alter the density of excitatory and inhibitory synapses labeled by the presynaptic markers vGLUT1 and vGAT, respectively (Fig. 3A).
The SynIE373K mutant does not affect excitatory and inhibitory synaptic density. A Representative images of SynIWT and SynIE373K transduced hippocampal neurons at 14 DIV triple stained for mCherry (red), vGLUT1 (gray), and vGAT (green). Nuclei were stained with Hoechst. Scale bar, 50 μm. B Excitatory synapses. Manders’ colocalization coefficient of vGLUT1- and mCherry-positive synapses (left) and linear density of vGLUT1-positive boutons on proximal dendrites of postsynaptic cells (right). C Inhibitory synapses. Manders’ colocalization coefficient of vGAT- and mCherry-positive synapses (left) and linear density of vGAT-positive boutons on proximal dendrites of postsynaptic cells (right). Data are shown as box plots. Synaptic density: n = 23 and 20 fields for SynIWT and SynIE373K, respectively; Manders’ colocalization coefficient: n = 18 and 30 fields for SynIWT and SynIE373K, respectively, from n = 3 independent cell culture preparations. p > 0.05; Mann–Whitney U-test/unpaired Student’s t-test
The analysis of the Manders’ colocalization coefficient revealed that about 50% of virally expressed Syn isoforms (mCherry-positive puncta) localized at vGLUT1- and vGAT-positive puncta identifying putative inhibitory and excitatory synaptic contacts (Fig. 3B, C; left panels). Next, we evaluated the number of excitatory and inhibitory synapses along proximal dendrites by vGLUT1/vGAT double immunostaining of transduced hippocampal neurons and found no significant differences in the density of both excitatory and inhibitory synaptic contacts (Fig. 3B, C; right panels). This demonstrates that the development of synaptic connectivity is not significantly affected by the E373K mutation and that the increased mEPSC frequency observed in SynIE373K transduced neurons can be tentatively attributed to an increased probability of spontaneous release.
SynIE373K increases the nerve terminal resting Ca2+ concentration
It is known that the spontaneous release of SVs that occurs stochastically in nerve terminals is dependent on the resting intraterminal [Ca2+]i [44, 45]. Thus, we addressed the possibility that SynI can actively bind and buffer Ca2+ in the nerve terminal under resting conditions, thus contributing to the resting [Ca2+]i. To ascertain whether deletion of the Ca2+-binding site on SynI alters resting [Ca2+]i, we co-transduced SynI KO hippocampal neurons with the SyGCaMP6f and either SynIWT or SynIE373K tagged with mCherry. SyGCaMP6f is an ultra-sensitive (Kd = 375 nM) genetically encoded fluorescent Ca2+ indicator in which GCaMP6f is fused to the cytoplasmic domain of the integral SV protein synaptophysin, allowing specific measurements of presynaptic Ca2+ concentrations in the nerve terminal cytosol surrounding SVs [63]. To effectively investigate the effects of SynIE373K expression on intraterminal resting Ca2+ concentrations, SyGCaMP6f fluorescence was quantitatively analyzed at mCherry-positive boutons (Fig. 4A, left).
Expression of the SynIE373K mutant is associated with an increase in resting, but not activity-stimulated, [Ca2+]i. A Nerve terminal Ca2+ measurements with SynGCaMP6. Primary SynI KO hippocampal neurons were co-transduced with SynGCaMP6 and either SynIWT or SynIE373K tagged with mCherry. Left: Representative images of mCherry-positive boutons where the fluorescence intensity was measured. Note the higher SynGCaMP6 fluorescence in boutons expressing SynIE373K. Scale bar, 6 μm. Middle: Mean (± SEM) integrated resting SynGCaMP6 fluorescence measured at mCherry-positive boutons in SynI KO neurons expressing either SynIWT (black, n = 14) or SynIE373K (blue, n = 14). Right: Mean (± SEM) integrated SynGCaMP6 fluorescence measured at mCherry-positive puncta in response to 2-s electrical field stimulation with 2–200 APs delivered at increasing frequencies (from 0.5 to 100 Hz). n = 15 and 14 fields for both SynIWT and SynIE373K from n = 3 independent neuronal preparations. *p < 0.05, unpaired Student’s t-test. B Nerve terminal Ca2+ measurements with Fura-2. Left: Representative images showing Fura-2 AM-filled neuronal branches from 14 DIV SynI KO hippocampal neurons transduced with either SynWT or SynIE373K fused to mCherry (red). Scale bar, 10 μm. Right: Box plots of basal Ca2+ concentrations, measured at mCherry-positive boutons. ***p < 0.001; Mann–Whitney U-test; n = 16 and n = 11 for SynIWT and SynIE373K, respectively, from n = 3 independent neuronal preparation
Interestingly, under resting conditions, boutons expressing SynIE373K displayed a significantly increased [Ca2+]i with respect to presynaptic sites expressing SynIWT (Fig. 4A, middle), indicating that SynIWT might function as a high-affinity Ca2+-buffer within nerve terminals. This possibility was also supported by the observation that no differences in the amplitude of activity-dependent Ca2+ transients were observed upon field stimulation in a wide range of stimulation frequencies (Fig. 4A, right). To independently assess the absolute [Ca2+]i at the presynaptic compartment, we loaded both SynIWT and SynIE373K transduced SynI KO neurons with the ratiometric Ca2+ sensor Fura-2 AM and analyzed Fura-2 fluorescence at the level of mCherry-positive puncta. Under resting conditions, SynIE373K-positive boutons showed a significant increase in the intraterminal [Ca2+]i compared to SynIWT-positive boutons (110.1 ± 18.78 nM vs 265.4 ± 30.02 nM; Fig. 4B), confirming the semiquantitative data obtained with SyGCaMP6f.
The effects of SynIE373K on miniature excitatory postsynaptic currents are rescued by intracellular Ca2+ chelators
The higher resting [Ca2+]i in SynIE373K-expressing neurons suggests that the increased frequency of mEPSCs, in the presence of unchanged synaptic density, is attributable to an increased probability of spontaneous release.
To obtain independent evidence on the mechanism and its specificity for excitatory synapses, we recorded mPSC events before and after the treatment with BAPTA-AM, a cell-permeable, fast Ca2+-chelator. Both excitatory and inhibitory mPSCs were initially recorded in the control extracellular solution for 2 min. After the addition of BAPTA-AM (2 µM) to the external solution, mPSCs were recorded for a further 15 min and analyzed in three consecutive time windows of 5 min. Strikingly, the increased mEPSC frequency that characterized SynI KO neurons transduced with SynIE373K was totally occluded by BAPTA-AM treatment, while mEPSC amplitude resulted similarly unchanged by BAPTA-AM in both genotypes (Fig. 5A). As expected, the BAPTA-AM treatment did not differentially affect SynIWT and SynIE373K expressing inhibitory synapses (Fig. 5B). Taken together, the data indicate that an alteration in the resting presynaptic Ca2+ homeostasis due to lack of Ca2+-binding to SynI could be the mechanistic cause of the specific enhancement of mEPSC frequency.
The enhancement of mEPSC frequency by the SynIE373K mutant is suppressed by BAPTA. A mEPSCs. Upper panels: Representative traces recorded at −70 mV in 14 DIV SynI KO hippocampal neurons transduced with either SynIWT (black) or SynIE373K (red) before and 5, 10, and 15 min after the addition of BAPTA-AM (2 μM). Lower panels: Time course of the mean (± SEM) changes in frequency (left) and amplitude (right) of mEPSCs. n = 5 and 8 for SynIWT and SynIE373K, respectively. B mIPSCs. Upper panels: Representative traces recorded at −70 mV in 14 DIV SynI KO hippocampal neurons transduced with either SynIWT (black) or SynIE373K (green) before and 5, 10, and 15 min after the addition of BAPTA-AM (2 μM). Lower panels: Time course of the mean (± SEM) changes in frequency (left) and amplitude (right) of mIPSCs. n = 12 and 14 for SynIWT and SynIE373K, respectively. Data are obtained from n = 3 independent neuronal preparations. *p < 0.05; two-way ANOVA/Bonferroni’s tests
SynIE373K reduces the amplitude of excitatory, but not inhibitory, evoked postsynaptic currents
Next, we investigated whether the expression of SynIE373K could differentially alter evoked neurotransmitter release by excitatory and inhibitory synapses. Since SynI plays pre- and post- docking roles in synaptic transmission [11, 50], we analyzed single-pulse evoked excitatory (eEPSCs) and inhibitory (eIPSCs) synaptic currents in SynIWT- and SynIE373K-expressing SynI KO neurons. eEPSCs were studied in primary autaptic neurons by depolarizing the cell membrane of the excitatory neuron to + 40 mV for 0.5 ms (Fig. 6A; left panel). Excitatory synapses expressing the SynIE373K mutant showed a significant reduction in the amplitude of eEPSCs in response to the first pulse with respect to SynIWT expressing synapses (Fig. 6A; right panel). The different amplitude of eEPSCs brought us to investigate the response to paired-pulse stimulation, in which both excitatory synapses were subjected to two consecutive stimuli at interstimulus intervals (ISI) ranging between 20 ms and 1 s (Fig. 6B; left panel). Excitatory synapses displayed facilitation at short ISIs that vanished at longer stimulation intervals. As previously reported [14, 70], SynIKO excitatory synapses displayed increased facilitation at short ISIs that was normalized by the expression of SynIWT. However, no differences in the expression of this short-term plasticity paradigm were found between SynIWT and SynIE373K (Fig. 6B, right panel).
Mutant SynIE373K decreases evoked EPSC, but not eIPSC, amplitude. A, B eEPSCs. A Left: Representative image of patched autaptic neuron for the study of eEPSCs at excitatory synapses. Scale bar, 15 μm. Right: Representative traces of single-pulse protocols applied to excitatory SynI KO synapses transduced with control vector (SynIKO, orange), SynIWT (black) or SynIE373K (red) and box plots of the eEPSC amplitude (n = 10, 14, 13 for SynIKO, SynIWT, and SynIE373K, respectively). B Left: Representative traces of paired-pulse protocols applied to excitatory synapses of the three genotypes. Right: Paired-pulse ratio (means ± SEM) of excitatory synapses transduced with control vector (SynIKO, orange), SynIWT (black), or SynIE373K (red) at interpulse intervals ranging from 20 ms to 1 s (n = 11–14, 20–21, 13–18 for SynIKO, SynIWT and SynIE373K, respectively). C, D eIPSCs. C Left: Representative images of low-density neuron culture for the study of eIPSCs at inhibitory synapses. Scale bar, 50 μm. Right: Representative traces of single-pulse protocols applied to inhibitory SynI KO synapses transduced with control vector (SynIKO, orange), SynIWT (black), or SynIE373K (green) and box plots of the eIPSC amplitude (n = 16, 13, 15 for SynIKO, SynIWT and SynIE373K, respectively). D Left: Representative traces of paired-pulse protocols applied to inhibitory synapses of the three genotypes. Right: Paired-pulse ratio (means ± SEM) of inhibitory synapses transduced with control vector (SynIKO, orange), SynIWT (black) or SynIE373K (red) at interpulse intervals ranging from 20 ms to 1 s (n = 16, 13, 15 for SynIKO, SynIWT and SynIE373K, respectively). Data are obtained from n = 3 independent neuronal preparations. *p < 0.05, **p < 0.01; Kruskal–Wallis/Dunn’s tests (A) or one-way ANOVA/Tukey’s tests (C). °p < 0.05 SynIE373K vs SynIKO; **p < 0.01 SynIWT vs SynIKO; two-way ANOVA/Tukey’s test (B, D)
eIPSCs were studied in low-density neuronal cultures, in which the presynaptic neuron was extracellularly stimulated by micromanipulating the stimulating electrode near the putative presynaptic neuron (Fig. 6C; left panel). No changes were observed in the amplitude of eIPSCs evoked in SynIE373K-transduced inhibitory synapses as compared with SynIWT synapses (Fig. 6C; right panel). Inhibitory synapses exhibited a characteristic depression in response to paired-pulse stimulation that was particularly intense at shorter ISIs and attenuated at longer stimulation intervals (Fig. 6D; left panel). This rescue from paired-pulse depression was closely similar in inhibitory synapses from the three genotypes (Fig. 6D; right panel).
The decreased amplitude of eEPSC responses indicates that the impaired Ca2+-buffering by mutated SynI under resting conditions does not play a role in the stimulus-dependent Ca2+ entry in the nanodomains of the active zones, suggesting the involvement of an alternative mechanism.
The reduction of eEPSC amplitude by SynIE373K is attributable to a decrease in the size of the RRPsyn
To identify the quantal parameters of release affected by SynIE373K mutation, we performed cumulative ePSC amplitude analysis in excitatory and inhibitory SynI KO neurons transduced with empty vector (SynIKO), SynIWT or SynIE373K. This method analyzes the cumulative amplitude profile during high- frequency trains of stimuli [5, 73], allowing to extract the values of Pr and RRPsyn.
When excitatory neurons were challenged with a 40 Hz train for 2 s, a significant depression of eEPSCs became apparent, irrespective of the amplitude of the first current in the train (Fig. 7A; left panel); the method assumes that Pr during the train approaches unity and that depression during the steady-state phase is limited by a constant recycling of SVs. Accordingly, the cumulative profile showed a rapid rise followed by a slower linear increase, reflecting the equilibrium between depletion and constant replenishment of the RRP (Fig. 7A; right panel). Consistent with previous reports [14], the eEPSC amplitude of SynIKO excitatory synapses was larger than that of SynIWT synapses, due to a pure increase in the RRPsyn size. The decreased eEPSC amplitude of the SynIE373K mutant (see also Fig. 6A) was entirely attributable to a significant drop, approximately of the same extent, in size of the RRPsyn, in the absence of changes in the Pr of evoked release (Fig. 7B) and consistent with the lack of effect on PPR at short time intervals (see Fig. 6B). To verify if the drop in both amplitude and RRPsyn of eEPSCs due to the expression of the SynIE373K mutant was strictly dependent on a presynaptic impairment or the result of a postsynaptic receptor deficit, we performed the same experiments in the presence of CTZ to selectively block AMPA receptor desensitization due to the intense glutamate release [15], Fig. 7C). Interestingly, the desensitization blockade did not change the quantal parameters reported above (Fig. 7D), confirming that the SynIE373K mutant negatively affects excitatory transmission by acting at the presynaptic level. The same cumulative amplitude analysis was performed on inhibitory synapses, using a 40 Hz train for 2.5 s (Fig. 7E). While the decreased eIPSC amplitude of SynIKO synapses was associated with a corresponding decrease in the RRPsyn, as previously reported [14], the lack of effect of SynIE373K on the eIPSC amplitude was confirmed by the absence of significant changes in the quantal parameters of synchronous inhibitory transmission (Fig. 7F). Taken together, the data suggest that the SynIE373K mutant affects the activity-dependent refilling of the RRP from the RecP specifically in excitatory synapses.
The SynIE373K mutant decreases the RRP size for synchronous release in excitatory, but not inhibitory, synapses. A–D Excitatory synapses. A, C Left: Representative traces showing the 2-s stimulation protocol @ 40 Hz used to estimate the quantal properties of synchronous release in the absence (A) or presence (C) of CTZ (60 µM) in excitatory SynI KO autapses expressing the control vector (SynIKO; orange), SynIWT (black) or SynIE373K (red). Right: The corresponding cumulative curves of the eEPSC amplitude during the stimulation train in the absence or presence of CTZ are shown as means ± SEM together with the linear regression fit to their linear phases (right). B, D Cumulative amplitude analysis of quantal parameters in excitatory autapses in the absence (B) or presence (D) of CTZ. Box plots of the amplitude of the first EPSC in the train (left), RRP size for synchronous release (RRPsyn; middle) and release probability (Pr; right). Without CTZ: n = 17, 29, 28; with CTZ: n = 12, 12, 9; for SynIKO SynIWT, and SynIE373K, respectively. E, F Inhibitory synapses. E Representative traces (left) showing the 2.5-s stimulation protocol @ 40 Hz used to estimate the quantal properties of synchronous release in inhibitory SynI KO synapses expressing the control vector (SynIKO; orange), SynIWT (black) or SynIE373K (green) and corresponding cumulative curves of the eIPSC amplitude during the stimulation train (right). F Box plots of the amplitude of the first IPSC in the train (left), RRP size for synchronous release (RRPsyn; middle) and release probability (Pr; right). n = 15, 16, 14 for SynIKO SynIWT, and SynIE373K, respectively. For further details, see “Materials and methods” section. Data are obtained from n = 3 independent neuronal preparations. *p < 0.05; **p < 0.01; ***p < 0.001; Kruskal–Wallis/Dunn’s tests (B) or one-way ANOVA/Tukey’s tests (D, F)
SynIE373K impairs the recovery from synaptic depression in both excitatory and inhibitory synapses
Central synapses in mice lacking one or more Syn isoforms display a marked decrease in SV density, reflecting a depletion of the RecP and changes in short-term, but not long-term, synaptic plasticity [34, 48, 69, 70, 76, 78, 80]. Accordingly, most of the available data indicate that Syn deletion enhances synaptic depression during repetitive stimulation [25, 34]. Excitatory and inhibitory synapses of SynI KO neurons transduced with either SynIWT or SynIE373K were subjected to a prolonged high-frequency stimulation (HFS). The progressive decay of the ePSCs amplitude during the train and the subsequent recovery from depression upon returning the stimulation frequency to 0.1 Hz were exponentially fitted and analyzed for the kinetic parameters and the steady-state current of depression and recovery. During sustained HFS (30 s @ 20 Hz), SynIE373K excitatory synapses presented no significant changes in the depression steady-state current (SSC) and in the slow and fast time constants of depression (Fig. 8A, B). However, they showed a significant impairment in the recovery after depression with an almost two-fold difference in the recovery SSC with respect to SynIWT, in the absence of changes in the time constant of recovery (Fig. 8A, C). Under sustained HFS (30 s @ 10 Hz), SynIE373K inhibitory synapses showed a significantly lower depression SSC than SynIWT inhibitory synapses, with no significant differences in the slow and fast time constants of depression (Fig. 8D, E). In addition, inhibitory synapses displayed an impaired recovery after depression similar to that observed at excitatory synapses: the first response after the stimulus train was markedly smaller than in SynIWT synapses and the recovery SSC was twofold lower, with no change in the time constant of recovery (Fig. 8D, F).
The SynIE373K mutant impairs recovery from depression in both excitatory and inhibitory synapses. A–C Excitatory synapses. Excitatory SynI KO autaptic neurons were transduced with either SynIWT (black) or SynIE373K (red). A Left: Representative traces of eEPSCs evoked by stimulating neurons with high-frequency trains lasting 30 s at 20 Hz. Right: The progressive decay of the eEPSC amplitude (means ± SEM) during the stimulation train and the subsequent recovery from depression upon return of the stimulation frequency to 0.1 Hz are plotted as a function of time from the beginning of the train (n = 9 and 8 for SynIWT and SynIE373K, respectively). B Box plots of the depression steady-state current (SSC, left) and of the fast (middle) and slow (right) time constants of depression (τ). C Box plots of the SSC (left) and τ (right) of recovery after the stimulation train. D–F Inhibitory synapses. Inhibitory SynI KO neurons were transduced with either SynIWT (black) or SynIE373K (green). D Left: Representative traces of eIPSCs evoked by stimulating neurons for 30 s at 20 Hz. Right: The progressive decay of the eIPSC amplitude (means ± SEM) during the stimulation train and the recovery from depression are plotted as a function of time (n = 9 and 8 for SynIWT and SynIE373K, respectively). (E) Box plots of the depression steady-state current (SSC, left) and of the fast (middle) and slow (right) time constants of depression (τ). (F) Box plots of the SSC (left) and τ (right) of recovery after the stimulation train. Data are from n = 3 independent neuronal preparations. *p < 0.05; **p < 0.01; Mann–Whitney U-test (A) or unpaired Student’s t-test (C–F)
These results suggest that the SynIE373K mutant strongly impairs the recovery from depression in both excitatory and inhibitory synapses and the steady state of depression in inhibitory synapses. This suggests that Ca2+ binding to SynI plays a role in the dynamics of SV pools and in SV mobilization during and after sustained HFS in both types of synapses.
SynIE373K impairs SV recycling and slows-down the kinetics of RRP refilling
To further investigate the mechanisms of defective recovery after the stimulus in SynIE373K synapses, we performed synaptophysin-pHluorin (sypHy)-based live cell imaging experiments. Primary SynI KO hippocampal neurons were co-transduced at 10 DIV with lentiviral vectors encoding sypHy and either SynIWT or SynIE373K fused to mCherry and analyzed 8–11 days after transduction (Fig. 9A).
The recovery from the synaptic activity is altered in nerve terminals expressing the SynIE373K mutant. A Representative images showing axonal branches from 21 DIV SynI KO hippocampal neurons transduced with sypHy (green) and either SynWT (left) or SynIE373K (right) fused to mCherry (red) during a 600 AP stimulation @ 20 Hz. White regions of interest (ROI) label co-transduced synapses. Scale bar, 3 µm. B Left: Ensemble average traces of sypHy fluorescence plotted for SynWT (black) and SynIE373K (blue). Stimulation (20 APs@100 Hz) is indicated by the gray bar. Data are means ± SEM of n = 7 and 11 coverslips for SynWT and SynIE373K, respectively. Middle: Box plot of peak fluorescence at the end of the stimulus in the two genotypes. Right: Box plot of time constant of endocytosis (τendo), evaluated by fitting the fluorescence decay after stimulation with a mono-exponential function. *p < 0.05, Mann–Whitney’s U-test. C Left: Representative traces from SynWT (black) and SynIE373K (blue) expressing synapses stimulated with three RRP trains (20 APs@100 Hz, thin gray bars), subjected to a long-lasting stimulation (600 APs@20 Hz, thick gray bar) and challenged with a further RRP stimulation. Traces were normalized to the first stimulus. Right: Box plot showing the average peak fluorescence for the three consecutive RRP stimulations before the long-lasting train, and the response to an additional RRP stimulation performed after the long-lasting train. Data are from n = 9 coverslips per genotype. *p < 0.05, one-way ANOVA/Tukey’s tests
We first evaluated the RRP, which in synaptic imaging accounts for both synchronous and asynchronous components of releasable SVs, by stimulating neurons with 20 APs @100 Hz [4]. While no genotype-dependent differences were present in the peak fluorescence, a significant increase in the time constant of fluorescence decay was observed in SynIE373K as compared to SynIWT terminals, revealing an impaired SV endocytosis, suggestive of defective recovery after RRP depletion (Fig. 9B). The lack of change in the RRP measured by sypHy imaging suggests that the decreased RRPsyn observed by patch-clamp recordings (see Fig. 7B) is accompanied by an increase of asynchronous release that may be facilitated by the increased resting Ca2+ (see Fig. 4). Next, to measure recovery after sustained stimulation, we performed repetitive RRP stimulations (20 APs @ 100 Hz) before or after a long-lasting stimulation (600 APs @ 20 Hz; Fig. 9C). Consecutive RRP stimuli resulted in a mild and not significant rundown in both SynIWT and SynIE373K synapses. Despite the peak fluorescence evoked by the first round of stimulation was not changed between the two genotypes, the RRP after the long-lasting stimulation was significantly reduced in SynIE373K synapses (Fig. 9C). These data confirmed the defective SV recovery after sustained stimulation in SynIE373K neurons.
Ultrastructural analysis reveals that SynIE373K affects SV trafficking at rest and during activity
To verify if the defects in the excitatory RRPsyn and recovery from synaptic depression in both excitatory and inhibitory synapses were due to alterations in SV trafficking, we evaluated the density and distribution of SVs in presynaptic terminals by electron microscopy of “asymmetric” (i.e., excitatory) and “symmetric” (i.e., inhibitory) synapses expressing either SynIWT or SynIE373K.
Transduced excitatory and inhibitory synapses were analyzed at rest (1 s before the stimulation train) and at the following HFS (30 s @ 20 Hz) by quickly fixing the samples at the end of train and during the recovery period (2 min after the train) (Fig. 10A, C). At rest, excitatory SynIE373K synapses showed a significant reduction in total SV density with respect to control SynIWT synapses (Fig. 10B; left panel). Although no genotype-dependent changes were detected in the density of physically docked SVs (Fig. 10B; right panel), the decreased SV content is consistent with an impaired RRP refilling in mutant nerve terminals that could account for the functionally determined decrease in RRPsyn (see Fig. 7B). The total SV density was decreased under the condition of deep synaptic depression reached at the end of the stimulation train, but with no apparent genotype-dependent differences. However, at the end of the recovery period, excitatory synapses failed to recover the basal SV density (Fig. 10B; left panel), recapitulating the defective recovery evaluated electrophysiologically (see Fig. 8A). At rest and the end of the stimulation train, inhibitory SynIE373K synapses were closely similar to control SynIWT synapses in terms of total and docked SV densities (Fig. 10D). However, similarly to excitatory synapses, the total SV density of mutant inhibitory terminals failed to recover the basal SV density, consistent with the electrophysiological data (Fig. 10D; left panel).
SV density and distribution are altered in excitatory and inhibitory synapses expressing the SynIE373K mutant. A, B Excitatory synapses. A Representative transmission electron micrographs of excitatory presynaptic terminals from SynI KO neurons transduced with either SynIWT (top) or SynIE373K (bottom) and rapidly (1 s) fixed under resting conditions (Pre), immediately after a 30 s tetanic stimulation @ 20 Hz (After train) and following 120 s of recovery (After recovery). Scale bar, 200 nm. B Morphometric analysis of excitatory presynaptic terminals. For each genotype and experimental condition, the total SV density (left) and the number of docked SVs (right) are shown as means ± SEM. C, D Inhibitory synapses. C Representative transmission electron micrographs of inhibitory presynaptic terminals quickly fixed under the conditions as described in A. Scale bar, 200 nm. D Morphometric analysis of inhibitory presynaptic terminals. The total SV density (left) and the number of docked SVs (right) are shown for each genotype and experimental condition as means ± SEM. Excitatory and inhibitory synapses were manually identified based on their asymmetric or symmetric shape. Excitatory synapses: n = 30 and 30; inhibitory synapses: n = 15 and 15; for SynIWT and SynIE373K, respectively, from 2 independent neuronal preparations. Across genotype: *p < 0.05; **p < 0.01; ***p < 0.001; unpaired Student’s t-test. Within genotype: °p < 0.05; °°°p < 0.001; vs “Pre” for SynIWT; +p < 0.05 vs “Pre” for SynIE373K; one-way ANOVA/Dunnett’s multiple comparison test
Taken together, these results show that the absence of Ca2+ binding to SynI mainly affects excitatory synapses that fail to maintain a correct distribution of SVs under basal conditions. Moreover, in both excitatory and inhibitory synapses, the deletion of the Ca2+ binding site dysregulates the SynI-mediated trafficking of SVs in terminals challenged with sustained HFS.
Discussion
Genetic analyses in human populations have identified several mutations in the SYN1 and SYN2 genes related to epilepsy and autism spectrum disorder [1, 17, 27, 31, 60, 65, 84], for a review, see [38]. Moreover, several studies of single and multiple Syn KO mice models confirmed the relationship between the Syn1/Syn2 genes, epilepsy, and autism [16, 26, 35, 48, 55, 70]. Thus, the study of the precise functional role of Syns in excitatory and inhibitory synapses and their structure–function relationships within nerve terminals is fundamental to understanding the pathogenesis of such diseases.
SynI is present in nerve terminals at an average concentration of about 10–20 µM. It represents the 4% of SV proteins and 8–10 copies of SynI are associated with a single SV [20, 72, 79]. The crystal structure of the highly conserved central C-domain of SynI revealed a binding site for ATP, formed by a flexible MFL loop (residues 330–343), with high similarity with “ATP-grasp” enzymes [23]. In the proximity of the ATP binding site, SynI binds Ca2+ by coordinating it with the pyrophosphate moiety of ATP and two glutamate residues (Glu373 and Glu386). Structural studies and biochemical data on recombinant Syns suggested the possibility that ATP binding is regulated by Ca2+ [39]. Notwithstanding these data, the role of ATP binding to SynI and SynII has remained elusive. Based on the crystal structure of domain C, an increase in SynI tetramer formation upon binding of ATP in the presence of Ca2+ was reported [10]. More recently, using MD simulations and biochemical assays, we found that the MFL loop has a similarly reduced motility to holo-SynI also in the absence of Ca2+ and, consistently, SynI can bind ATP also in the absence of Ca2+ [62]. We also found that ATP binding strengthens the SynI association with SVs, favors the formation of high-order dimers and tetramers, and increases SV clustering. Under these conditions, the presence of Ca2+ negatively modulates SV clustering and favors the formation of tetramers over that of dimers [62] consistent with the previous structural predictions [10].
Deletion of ATP binding in SynI induced an increase in the probability of release at inhibitory synapses that potentiated the responses to single stimulation and increased synaptic depression [62]. Deletion of the same site in SynII also increased the probability of release and synaptic depression at excitatory synapses [75]. Although the two studies were done in distinct types of synapses, they show a similar functional outcome of the ATP binding site deletion in both Syn isoforms irrespective of the presence of Ca2+ binding, indicating the existence of distinct roles for Ca2+ and ATP binding to SynI/SynII. Since the micromolar ATP concentrations in nerve terminals do not represent a limiting factor for protein binding [67], it has so far been difficult to investigate separately the role of ATP binding to SynI from that of Ca2+ binding, and no specific role for Ca2+ binding to SynI has been proposed, except for facilitating ATP binding. Here, we show that ATP and Ca2+ binding by the SynI C-domain are independent events that, however, cooperatively potentiate each other. Thus, Ca2+ enhances ATP binding and, conversely, ATP increases the strength of Ca2+binding to SynI. To dissect out the physiological role of Ca2+ binding to SynI, we analyzed the effects of the E373K mutation that deletes the major Ca2+ binding site in SynI and induces a destabilization of the SynI oligomerization interface that is not dissimilar to that observed after deletion of the ATP binding site [62].
We found that the ablation of the Ca2+ binding in SynI had more severe consequences in excitatory than in inhibitory synapses. In the former synapses, the deletion of SynI-Ca2+ binding increased the frequency of mEPSCs without altering synaptic density and decreased the EPSC amplitude evoked by single stimuli. Distinct, but related, mechanisms are involved in these two effects. Under physiological conditions, SynI may act as a Ca2+ buffer, regulating the concentration of this ion in the presynaptic compartment. In the absence of Ca2+ sequestration by SynI, the resting Ca2+ concentration in excitatory nerve terminals could increase sufficiently to enhance spontaneous SV exocytosis that generates miniature events. Indeed, although the spontaneous release is independent of action potentials, it is at least partly Ca2+-dependent [44, 45, 59, 77]. This hypothesis is further confirmed by the observation that supplementation of an exogenous Ca2+ buffer completely occluded the effect of the SynI mutant. Why a similar effect is not occurring in inhibitory synapses that also express, to a large extent, SynI [9]? One possible explanation is that inhibitory neurons express a variety of Ca2+-buffering proteins [74], such as parvalbumin, calbindin, and calretinin [3, 22, 37, 41, 46, 77, 81] that characterize their peculiar functional properties. Under these conditions, the expression of Ca2+-buffering proteins by distinct classes of interneurons may render the contribution of the SynI-mediated buffering dispensable under resting conditions. It will be interesting to investigate whether the expression of parvalbumin in excitatory neurons occludes the effect of SynIE373K mutation or, alternatively, whether silencing endogenous Ca2+ buffers in inhibitory neurons makes them sensitive to the E373K mutation.
On the other hand, the decreased EPSC amplitude due to a selective decrease in RRP size can be a consequence of the decreased SV density observed by electron microscopy in excitatory synapses under resting conditions. Although we did not detect any change in the density of physically docked SVs, the decrease in RRP can be explained by a decreased refilling with primed SVs from a more dispersed RecP. Such dispersion can be a consequence of: (i) the increased resting intraterminal Ca2+ that stimulates CaM kinases to phosphorylate SynI, favoring the release of SVs from the clusters and the actin cytoskeleton; and/or (ii) a possible mutation-induced change in SynI oligomerization, as suggested by MD simulations, that may alter the SV clustering capacity. Recent work demonstrated the capability of Syns to trigger liquid phase separation that participates in the clustering and maintenance of the SV pools in nerve terminals [57, 58, 64] and that is released upon Syn phosphorylation by CaM kinases [58]. Thus, a tonic activation of CaM kinases due to the increased concentration of resting Ca2+ could also impair SynI liquid phase separation and lead to SV cluster dispersion. While the deletion of the Ca2+ binding site in SynI can be defined as a loss-of-function, some of the phenotypes of SynIE373K appear as gain-of-function when compared to SynIWT and SynIKO. Indeed, SynIE373K induces an increase in mEPSC frequency, as the consequence of the increase in resting free Ca2+, while constitutive depletion of SynI does not affect mEPSC frequency or amplitude [14]. On the other hand, constitutive deletion of SynI increases eEPSC amplitude and RRP size as a secondary effect of the impairment in inhibitory transmission [14, 25, 82], while SynIE373K behaves similarly to SynIWT, indicating that the RRP size does not depend on Ca2+ binding to SynI.
Another effect of the deletion of Ca2+-binding to SynI, shared by excitatory and inhibitory synapses, consists of a sharp impairment in recovering PSCs after prolonged HFS. This effect finds its ultrastructural counterpart in a marked depletion of SVs at the end of the recovery period, indicating a defective RRP refilling. This effect, together with the impaired and slowed down retrieval of exocytosed SVs, suggests a modulation exerted by Ca2+ binding on the kinetics of endocytosis that could be mediated by the previously reported interactions of SynI with endocytic proteins such as intersectin and amphiphysin [24, 32, 61]. While all the remaining effects of the Syn mutant were exclusively found in facilitating excitatory synapses, the effects on recovery from depression also extended to an inhibitory transmission that is more prone to experience depression given its higher probability of release.
The putative events triggered by the deletion of Ca2+ binding to SynI in excitatory synapses are schematically represented in Fig. 11. Inability to bind Ca2+ increases the resting Ca2+ concentrations which, in turn, increases the frequency of mEPSCs and constitutively activates CaM kinases phosphorylating SynI at distinct sites and promoting its dissociation from SVs and actin. This will in turn reduce SV clustering and disperse SVs in the RecP. The impaired SV clustering may be also contributed by the effect of the mutation on SynI oligomerization [62]. The dispersion of SVs in the RecP can, in turn, be responsible for the decreased RRPsyn and eEPSC amplitude, possibly due to a decreased SV priming and/or refilling of RRP, and for the marked impairment of EPSC recovery after prolonged HFS.
Schematic model of the functional consequences of the lack of Ca2+-binding to SynI in excitatory synapses. The lack of Ca2+-buffering by SynI in excitatory synapses, characterized by a low Ca2+-buffering capacity, results in an increased intraterminal [Ca2+]i under resting conditions. This increases the probability of spontaneous SV fusion events and decreases the clustering of SVs in the RecP. This results in a decrease in RRP size for synchronous release and an impairment of the reconstitution of SV stores during recovery after HFS. The decrease in SV clustering may be contributed by an increased tetramerization of SynI and/or to increased phosphorylation of SynI by CaM kinases induced by the rise of resting intraterminal [Ca2+]i. Inhibitory synapses, endowed with a more efficient Ca2+-buffering capacity, are only affected by the lack of Ca2+-buffering by SynI under the intense Ca2+ build-up that occurs during HFS
For the time being, the E373K mutation has not been identified in the SYN1 gene of patients suffering from autism spectrum disorders or epilepsy. The first nonsense mutation in SYN1 to be reported (W356X; [31] also deletes the Ca2+ binding site; however, the expression of the truncated form of synapsin I was markedly impaired, due to both the strongly decreased translation by non-sense mediated RNA decay (NMD) and severe intracellular aggregation of the NMD-escaped protein [33].
In conclusion, we have shown that Ca2+ binding to SynI is fundamental for maintaining a physiological SV organization at rest and during/after HFS. Excitatory neurons seem to be more sensitive to the Ca2+-dependent activity of SynI, indicating a putative role of SynI as Ca2+ buffer in these terminals under resting conditions. These findings contribute to the clarification of the distinct roles of ATP and Ca2+ binding to the highly conserved central C-domain of Syns and underline the central role of the regulation of Ca2+ basal levels and transients in nerve terminals in driving SV trafficking, synaptic transmission, and plasticity.
Availability of data and material
The data and material that support the findings of this study are available upon request to the corresponding authors.
References
Accogli A, Wiegand G, Scala M, Cerminara C, Iacomino M, Riva A, Carlini B, Camerota L, Belcastro V, Prontera P, Fernández-Jaén A, Bebek N, Scudieri P, Baldassari S, Salpietro V, Novelli G, De Luca C, von Stülpnagel C, Kluger F, Kluger GJ, Wohlrab GC, Ramantani G, Lewis-Smith D, Thomas RH, Lai M, Verrotti A, Striano S, Depienne C, Minetti C, Benfenati F, Brancati F, Zara F, Striano P (2021) Clinical and genetic features in patients with reflex bathing epilepsy. Neurology 97:e577–e586
Alabi AA, Tsien RW (2012) Synaptic vesicle pools and dynamics. Cold Spring Harb Perspect Biol 4:a013680
Aponte Y, Bischofberger J, Jonas P (2008) Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J Physiol 586:2061–2075
Ariel P, Ryan TA (2010) Optical mapping of release properties in synapses. Front Neural Circuits 4:18
Baldelli P, Fassio A, Valtorta F, Benfenati F (2007) Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci 27:13520–13531
Bekkers JM, Stevens CF (1991) Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA 88:7834–7838
Benfenati F, Valtorta F, Chieregatti E, Greengard P (1992) Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron 8:377–386
Benfenati F, Valtorta F, Rossi MC, Onofri F, Sihra T, Greengard P (1993) Interactions of synapsin I with phospholipids: possible role in synaptic vesicle clustering and in the maintenance of bilayer structures. J Cell Biol 123:1845–1855
Bragina L, Candiracci C, Barbaresi P, Giovedì S, Benfenati F, Conti F (2007) Heterogeneity of glutamatergic and GABAergic release machinery in cerebral cortex. Neuroscience 146:1829–1840
Brautigam CA, Chelliah Y, Deisenhofer J (2004) Tetramerization and ATP binding by a protein comprising the A, B, and C domains of rat synapsin I. J Biol Chem 279:11948–11956
Cesca F, Baldelli P, Valtorta F, Benfenati F (2010) The synapsins: key actors of synapse function and plasticity. Prog Neurobiol 91:313–348
Chi P, Greengard P, Ryan TA (2001) Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4:1187–1193
Chi P, Greengard P, Ryan TA (2003) Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 38:68–78
Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, Martinoia S, Benfenati F (2009) Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb Cortex 19:1422–1439
Coombs ID, Soto D, McGee TP, Gold MG, Farrant M, Cull-Candy SG (2019) Homomeric GluA2(R) AMPA receptors can conduct when desensitized. Nat Commun 10:4312
Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M, Benfenati F (2008) Synapsin I and synapsin II null mice display an increased age-dependent cognitive impairment. J Cell Sci 121:3042–3051
Corradi A, Fadda M, Piton A, Patry L, Marte A, Rossi P, Cadieux-Dion M, Gauthier J, Lapointe L, Mottron L, Valtorta F, Rouleau GA, Fassio A, Benfenati F, Cossette P (2014) SYN2 is an autism predisposing gene: loss-of-function mutations alter synaptic vesicle cycling and axon outgrowth. Hum Mol Genet 23:90–103
Crawford DC, Kavalali ET (2015) Molecular underpinnings of synaptic vesicle pool heterogeneity. Traffic 16:338–364
Darden T, York D, Pedersen L (1993) Particle mesh Ewald: a Nlog(N) method for Ewald sums in large systems. J Chem Phys 98:10089–10092
De Camilli P, Benfenati F, Valtorta F, Greengard P (1990) The synapsins. Annu Rev Cell Biol 6:433–460
De Palma M, Naldini L (2002) Transduction of a gene expression cassette using advanced generation lentiviral vectors. Methods Enzymol 346:514–529
Eggermann E, Jonas P (2012) How the ‘slow’ Ca2+ buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci 15:20–22
Esser L, Wang CR, Hosaka M, Smagula CS, Südhof TC, Deisenhofer J (1998) Synapsin I is structurally similar to ATP-utilizing enzymes. EMBO J 17:977–984
Evergren E, Benfenati F, Shupliakov O (2007) The synapsin cycle: a view from the synaptic endocytic zone. J Neurosci Res 85:2648–2656
Farisello P, Boido D, Nieus T, Medrihan L, Cesca F, Valtorta F, Baldelli P, Benfenati F (2013) Synaptic and extrasynaptic origin of the excitation/inhibition imbalance in the hippocampus of 785 synapsin I/II/III knockout mice. Cereb Cortex 23:581–593
Fassio A, Raimondi A, Lignani G, Benfenati F, Baldelli P (2011) Synapsins: from synapse to network hyperexcitability and epilepsy. Semin Cell Dev Biol 22:408–415
Fassio A, Patry L, Congia S, Onofri F, Piton A, Gauthier J, Pozzi D, Messa M, Defranchi E, Fadda M, Corradi A, Baldelli P, Lapointe L, St-Onge J, Meloche C, Mottron L, Valtorta F, Khoa Nguyen D, Rouleau GA, Benfenati F, Cossette P (2011) SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum Mol Genet 20:2297–2307
Fornasiero EF, Bonanomi D, Benfenati F, Valtorta F (2010) The role of synapsins in neuronal development. Cell Mol Life Sci 67:1383–1396
Forte N, Binda F, Contestabile A, Benfenati F, Baldelli P (2020) Synapsin I synchronizes GABA release in distinct interneuron subpopulations. Cereb Cortex 30:1393–1406
Frenkel D, Smit B (2001) Understanding molecular simulation: from algorithms to applications, 2nd edn. Academic Press, San Diego
Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship JA (2004) Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet 41:183–186
Gerth F, Jäpel M, Pechstein A, Kochlamazashvili G, Lehmann M, Puchkov D, Onofri F, Benfenati F, Nikonenko AG, Fredrich K, Shupliakov O, Maritzen T, Freund C, Haucke V (2017) Intersectin associates with synapsin and regulates its nanoscale localization and function. Proc Natl Acad Sci USA 114:12057–12062
Giannandrea M, Guarnieri FC, Gehring NH, Monzani E, Benfenati F, Kulozik AE, Valtorta F (2013) Nonsense-mediated mRNA decay and loss-of-function of the protein underlie the X-linked epilepsy associated with the W356X mutation in synapsin I. PLoS ONE 8:e67724
Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguez RM, Wetsel WC, Greengard P, Augustine GJ (2004) Different presynaptic roles of synapsin at excitatory and inhibitory synapses. J Neurosci 24:11368–11380
Greco B, Managò F, Tucci V, Kao HT, Valtorta F, Benfenati F (2013) Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res 251:65–74
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Gulyás AI, Hájos N, Freund TF (1996) Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci 16:3397–3411
Guerrini R, Conti V, Mantegazza M, Balestrini S, Galanopoulou AS, Benfenati F (2022) Developmental and epileptic encephalopathies: from genetic heterogeneity to phenotypic continuum. Physiol Rev. Epub ahead of print. https://doi.org/10.1152/physrev.00063.2021
Hosaka M, Südhof TC (1998) Synapsin I and II are ATP-binding proteins with differential Ca2+ regulation. J Biol Chem 273:1425–1429
Hosaka M, Südhof TC (1999) Homo- and heterodimerization of synapsins. J Biol Chem 274:16747–16753
Hu H, Gan J, Jonas P (2014) Fast spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science 345:1255263–1255263
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC Jr, Greengard P, Czernik AJ (2001) Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci 21:7944–7953
Kavalali ET (2015) The mechanisms and functions of spontaneous neurotransmitter release. Nat Rev Neurosci 16:5–16
Kavalali ET (2020) Neuronal Ca2+ signalling at rest and during spontaneous neurotransmission. J Physiol 598:1649–1654
Lee SH, Rosenmund C, Schwaller B, Neher E (2000) Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. J Physiol 525:405–418
Leung A (1994) Fixation. In: Woods AE, Ellis RC (eds) Laboratory histopathology: a complete reference. Churchill Livingstone, New York
Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P, Anderson P (1995) Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA 92:9235–9239
Li M, Wang Y, Banerjee R, Marinelli F, Silberberg S, Faraldo-Gómez JD, Hattori M, Swartz KJ (2019) Molecular mechanisms of human P2X3 receptor channel activation and modulation by divalent cation bound ATP. Elife 8:e47060
Longhena F, Faustini G, Brembati V, Pizzi M, Benfenati F, Bellucci A (2021) An updated reappraisal of synapsins: structure, function and role in neurological and psychiatric disorders. Neurosci Biobehav Rev 130:33–60
Mackerell AD Jr, Feig M, Brooks CL 3rd (2004) Extending the treatment of backbone energetics in protein force fields: limitations of gas-phase quantum mechanics in reproducing protein conformational distributions in molecular dynamics simulations. J Comput Chem 25:1400–1415
Martyna GJ, Tobias DJ, Klein ML (1994) Constant pressure molecular dynamics algorithms. J Chem Phys 101:4177–4189
Medrihan L, Cesca F, Raimondi A, Lignani G, Baldelli P, Benfenati F (2013) Synapsin II desynchronizes neurotransmitter release at inhibitory synapses by interacting with presynaptic calcium channels. Nat Commun 4:1512
Medrihan L, Ferrea E, Greco B, Baldelli P, Benfenati F (2015) Asynchronous GABA release is a key determinant of tonic inhibition and controls neuronal excitability: a study in the synapsin II-/- mouse. Cereb Cortex 25:3356–3368
Michetti C, Caruso A, Pagani M, Sabbioni M, Medrihan L, David G, Galbusera A, Morini M, Gozzi A, Benfenati F, Scattoni ML (2017) The knockout of synapsin II in mice impairs social behavior and functional connectivity generating an ASD-like phenotype. Cereb Cortex 27:5014–5023
Michetti C, Falace A, Benfenati F, Fassio A (2022) Synaptic genes and neurodevelopmental disorders: from molecular mechanisms to developmental strategies of behavioral testing. Neurobiol Dis 173:105856
Milovanovic D, De Camilli P (2017) Synaptic vesicle clusters at synapses: a distinct liquid phase? Neuron 93:995–1002
Milovanovic D, Wu Y, Bian X, De Camilli P (2018) A liquid phase of synapsin and lipid vesicles. Science 361:604–607
Neher E, Sakaba T (2008) Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59:861–872
Nguyen DK, Rouleau I, Sénéchal G, Ansaldo AI, Gravel M, Benfenati F, Cossette P (2015) X-linked focal epilepsy with reflex bathing seizures: characterization of a distinct epileptic syndrome. Epilepsia 56:1098–1108
Onofri F, Giovedi S, Kao HT, Valtorta F, Bongiorno Borbone L, De Camilli P, Greengard P, Benfenati F (2000) Specificity of the binding of synapsin I to Src homology 3 domains. J Biol Chem 275:29857–29867
Orlando M, Lignani G, Maragliano L, Fassio A, Onofri F, Baldelli P, Giovedí S, Benfenati F (2014) Functional role of ATP binding to synapsin I in synaptic vesicle trafficking and release dynamics. J Neurosci 34:14752–14768
Orlando M, Schmitz D, Rosenmund C, Herman MA (2019) Calcium-independent exo-endocytosis coupling at small central synapses. Cell Rep 29:3767-3774.e3
Pechstein A, Tomilin N, Fredrich K, Vorontsova O, Sopova E, Evergren E, Haucke V, Brodin L, Shupliakov O (2020) Vesicle clustering in a living synapse depends on a synapsin region that 96 mediates phase separation. Cell Rep 30:2594–2602
Peron A, Baratang NV, Canevini MP, Campeau PM, Vignoli A (2018) Hot water epilepsy and SYN1 variants. Epilepsia 59:2162–2163
Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K (2005) Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802
Rangaraju V, Calloway N, Ryan TA (2014) Activity-driven local ATP synthesis is required for synaptic function. Cell 156:825–835
Rizzoli SO (2014) Synaptic vesicle recycling: steps and principles. EMBO J 33:788–822
Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Südhof TC (1993) Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell 75:661–670
Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC (1995) Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375:488–493
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) Numerical integration of the Cartesian equations of motion of a system with constraints and molecular dynamics of n-alkanes. J Comput Phys 23:327–341
Schiebler W, Jahn R, Doucet JP, Rothlein J, Greengard P (1986) Characterization of synapsin I binding to small synaptic vesicles. J Biol Chem 261:8383–8390
Schneggenburger R, Meyer AC, Neher E (1999) Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 23:399–409
Schwaller B (2010) Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol 2:a004051
Shulman Y, Stavsky A, Fedorova T, Mikulincer D, Atias M, Radinsky I, Kahn J, Slutsky I, Gitler D (2015) ATP binding to synapsin IIa regulates usage and clustering of vesicles in terminals of hippocampal neurons. J Neurosci 35:985–998
Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Triller A, Marty S (2007) Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci 27:6868–6877
Simkus CR, Stricker C (2002) The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol 545:521–535
Spillane DM, Rosahl TW, Südhof TC, Malenka RC (1995) Long-term potentiation in mice lacking synapsins. Neuropharmacology 34:1573–1579
Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R (2006) Molecular anatomy of a trafficking organelle. Cell 127:831–846
Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T, Takahashi T, Hirokawa N (1995) Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J Cell Biol 131:1789–1800
Tremblay R, Lee S, Rudy B (2016) GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91:260–292
Valente P, Farisello P, Valtorta F, Baldelli P, Benfenati F (2017) Impaired GABAB-mediated presynaptic inhibition increases excitatory strength and alters short-term plasticity in synapsin knockout mice. Oncotarget 8:90061–90076
Verstegen AM, Tagliatti E, Lignani G, Marte A, Stolero T, Atias M, Corradi A, Valtorta F, Gitler D, Onofri F, Fassio A, Benfenati F (2014) Phosphorylation of synapsin I by cyclin-dependent kinase-5 sets the ratio between the resting and recycling pools of synaptic vesicles at hippocampal synapses. J Neurosci 34:7266–7280
Xiong J, Duan H, Chen S, Kessi M, He F, Deng X, Zhang C, Yang L, Peng J, Yin F (2021) Familial SYN1 variants related neurodevelopmental disorders in Asian pediatric patients. BMC Med Genomics 14:182
Acknowledgements
We thank Dr. Anneke Verstegen (Istituto Italiano di Tecnologia, Genova, Italy) for the generation of the mutant SYN1 cDNA; prof. Luigi Naldini (Tiget, Milano, Italy) for kindly providing the lentiviral plasmids; the Viral Core Facility of Charité (Universitätsmedizin Berlin, Germany) (for the supply of the SynGCaMP6 viral vector; Drs. Riccardo Navone, Arta Mehilli, and Diego Moruzzo (Istituto Italiano di Tecnologia, Genova, Italy) for their help in the maintenance, breeding, and genotyping of the SynI KO mouse strain.
Funding
Open access funding provided by Istituto Italiano di Tecnologia within the CRUI-CARE Agreement. The study was supported by research grants from Compagnia di San Paolo Torino (2015.0546 and 2020.34760 to FB and 2017.20612 to PB); IRCCS Ospedale Policlinico San Martino (Ricerca Corrente and "5 × 1000" to PB and FB) and Italian Ministry of University and Research (PRIN 2015-H4K2CR and 2017-A9MK4R to FB). The support of Telethon-Italy (Grant GGP19120 to FB) is also acknowledged.
Author information
Authors and Affiliations
Contributions
MM, GL and TR performed and analyzed the electrophysiological experiments; SC prepared the low-density and autaptic neuronal cultures; MO and ADF performed and analyzed the electron microscopy experiments; DA performed live imaging experiments; LM performed and analyzed MD simulations; AF planned and supervised the live imaging experiments; PB, supervised the electrophysiological experiments and data analysis; FB planned and funded the research, contributed to data analysis and interpretation and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experiments were carried out in accordance with the guidelines established by the European Community Council (Directive 2010/63/EU of 22 September 2010) and were approved by the Italian Ministry of Health (authorization n. 605/2021-PR).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moschetta, M., Ravasenga, T., De Fusco, A. et al. Ca2+ binding to synapsin I regulates resting Ca2+ and recovery from synaptic depression in nerve terminals. Cell. Mol. Life Sci. 79, 600 (2022). https://doi.org/10.1007/s00018-022-04631-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04631-5