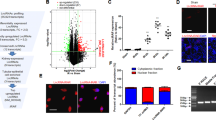
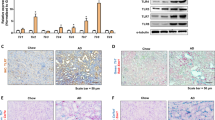
Abstract
Increasing evidence shows that long non-coding RNAs (lncRNAs) play an important role in a variety of disorders including kidney diseases. It is well recognized that inflammation is the initial step of kidney injury and is largely mediated by nuclear factor Kappa B (NF-κB) signaling. We had previously identified lncRNA-Arid2-IR is an inflammatory lncRNA associated with NF-κB-mediated renal injury. In this study, we examined the regulatory mechanism through which Arid2-IR activates NF-κB signaling. We found that Arid2-IR was differentially expressed in response to various kidney injuries and was induced by transforming growth factor beta 1(TGF-β1). Using RNA sequencing and luciferase assays, we found that Arid2-IR regulated the activity of NF-κB signal via NLRC5-dependent mechanism. Arid2-IR masked the promoter motifs of NLRC5 to inhibit its transcription. In addition, during inflammatory response, Filamin A (Flna) was increased and functioned to trap Arid2-IR in cytoplasm, thereby preventing its nuclear translocation and inhibition of NLRC5 transcription. Thus, lncRNA Arid2-IR mediates NF-κB-driven renal inflammation via a NLRC5-dependent mechanism and targeting Arid2-IR may be a novel therapeutic strategy for inflammatory diseases in general.










Similar content being viewed by others
Abbreviations
- UUO:
-
Unilateral ureteral obstruction
- mTEC:
-
Primary mouse tubular epithelial cells
- DKD:
-
Diabetic kidney disease
- IRI:
-
Ischemia Reperfusion Injury
- SSC:
-
Saline Sodium Citrate
- PBS:
-
Phosphate-buffered saline
- Flna:
-
Filamin A
- NLRC5:
-
NLR family CARD domain containing 5
- qPCR:
-
Quantitative real-time PCR
- RIP:
-
RNA immunoprecipitation
- DEGs:
-
Differentially expressed genes
References
Courtois G, Gilmore TD (2006) Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25:6831–6843
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663
Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Liu Z, Su B (2001) The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol 2:620–624
Annemann M, Plaza-Sirvent C, Schuster M, Katsoulis-Dimitriou K, Kliche S, Schraven B, Schmitz I (2016) Atypical IkappaB proteins in immune cell differentiation and function. Immunol Lett 171:26–35
Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annu Rev Biochem 81:145–166
Banfai B, Jia H, Khatun J, Wood E, Risk B, Gundling WE Jr, Kundaje A, Gunawardena HP, Yu Y, Xie L et al (2012) Long noncoding RNAs are rarely translated in two human cell lines. Genome Res 22:1646–1657
Ulitsky I, Bartel DP (2013) lincRNAs: genomics, evolution, and mechanisms. Cell 154:26–46
Schmitt AM, Chang HY (2016) Long noncoding RNAs in cancer pathways. Cancer Cell 29:452–463
Kolling M, Genschel C, Kaucsar T, Hubner A, Rong S, Schmitt R, Sorensen-Zender I, Haddad G, Kistler A, Seeger H et al (2018) Hypoxia-induced long non-coding RNA Malat1 is dispensable for renal ischemia/reperfusion-injury. Scientific reports 8:3438
Liu X, Hong C, Wu S, Song S, Yang Z, Cao L, Song T, Yang Y (2019) Downregulation of lncRNA TUG1 contributes to the development of sepsis-associated acute kidney injury via regulating miR-142–3p/sirtuin 1 axis and modulating NF-kappaB pathway. J Cell Biochem 120(7):11331–11341
Hu M, Wang R, Li X, Fan M, Lin J, Zhen J, Chen L, Lv Z (2017) LncRNA MALAT1 is dysregulated in diabetic nephropathy and involved in high glucose-induced podocyte injury via its interplay with beta-catenin. J Cell Mol Med 21:2732–2747
Li X, Zeng L, Cao C, Lu C, Lian W, Han J, Zhang X, Zhang J, Tang T, Li M (2017) Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res 350:327–335
Duan LJ, Ding M, Hou LJ, Cui YT, Li CJ, Yu DM (2017) Long noncoding RNA TUG1 alleviates extracellular matrix accumulation via mediating microRNA-377 targeting of PPARgamma in diabetic nephropathy. Biochem Biophys Res Commun 484:598–604
Alvarez ML, DiStefano JK (2011) Functional characterization of the plasmacytoma variant translocation 1 gene (PVT1) in diabetic nephropathy. PLoS ONE 6:e18671
Hanson RL, Craig DW, Millis MP, Yeatts KA, Kobes S, Pearson JV, Lee AM, Knowler WC, Nelson RG, Wolford JK (2007) Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes 56:975–983
Zhou Q, Chung AC, Huang XR, Dong Y, Yu X, Lan HY (2014) Identification of novel long noncoding RNAs associated with TGF-beta/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol 184:409–417
Zhou Q, Huang XR, Yu J, Yu X, Lan HY (2015) Long noncoding RNA Arid2-IR is a novel therapeutic target for renal inflammation. Mol Ther 23:1034–1043
Kopp F, Mendell JT (2018) Functional classification and experimental dissection of long noncoding RNAs. Cell 172:393–407
Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, Wei S, Zhao L, Vatan L, Wen B et al (2018) Aerobic glycolysis controls myeloid-derived suppressor cells and tumor immunity via a specific CEBPB isoform in triple-negative breast cancer. Cell Metab 28:87–103.e106
Gao Y, Sun W, Shang W, Li Y, Zhang D, Wang T, Zhang X, Zhang S, Zhang Y, Yang R (2018) Lnc-C/EBPbeta negatively regulates the suppressive function of myeloid-derived suppressor cells. Cancer Immunol Res 6:1352–1363
Wu Y, Shi T, Li J (2019) NLRC5: a paradigm for NLRs in immunological and inflammatory reaction. Cancer Lett 451:92–99
Yoshihama S, Roszik J, Downs I, Meissner TB, Vijayan S, Chapuy B, Sidiq T, Shipp MA, Lizee GA, Kobayashi KS (2016) NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc Natl Acad Sci USA 113:5999–6004
Luan P, Jian W, Xu X, Kou W, Yu Q, Hu H, Li D, Wang W, Feinberg MW, Zhuang J et al (2019) NLRC5 inhibits neointima formation following vascular injury and directly interacts with PPARgamma. Nat Commun 10:2882
Quinn JJ, Chang HY (2016) Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17:47–62
Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS (2001) Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol 2:138–145
Zhou Q, Xiong Y, Huang XR, Tang P, Yu X, Lan HY (2015) Identification of genes associated with Smad3-dependent renal injury by RNA-seq-based transcriptome analysis. Sci Rep 5:17901
Deng W, Lopez-Camacho C, Tang JY, Mendoza-Villanueva D, Maya-Mendoza A, Jackson DA, Shore P (2012) Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc Natl Acad Sci USA 109:1524–1529
Kobayashi KS, van den Elsen PJ (2012) NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol 12:813–820
Meissner TB, Li A, Kobayashi KS (2012) NLRC5: a newly discovered MHC class I transactivator (CITA). Microbes Infect 14:477–484
Staehli F, Ludigs K, Heinz LX, Seguin-Estevez Q, Ferrero I, Braun M, Schroder K, Rebsamen M, Tardivel A, Mattmann C et al (2012) NLRC5 deficiency selectively impairs MHC class I- dependent lymphocyte killing by cytotoxic T cells. J Immun (Baltimore, Md.:1950) 188:3820–3828
Cui J, Zhu L, Xia X, Wang HY, Legras X, Hong J, Ji J, Shen P, Zheng S, Chen ZJ et al (2010) NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell 141:483–496
Yao Y, Wang Y, Chen F, Huang Y, Zhu S, Leng Q, Wang H, Shi Y, Qian Y (2012) NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res 22:836–847
Li Q, Wang Z, Zhang Y, Zhu J, Li L, Wang X, Cui X, Sun Y, Tang W, Gao C et al (2018) NLRC5 deficiency protects against acute kidney injury in mice by mediating carcinoembryonic antigen-related cell adhesion molecule 1 signaling. Kidney Int 94:551–566
Nakamura F, Stossel TP, Hartwig JH (2011) The filamins: organizers of cell structure and function. Cell Adhes Migr 5:160–169
Shao QQ, Zhang TP, Zhao WJ, Liu ZW, You L, Zhou L, Guo JC, Zhao YP (2016) Filamin A: insights into its exact role in cancers. Pathol Oncol Res POR 22:245–252
Wang J, Zhao S, Wei Y, Zhou Y, Shore P, Deng W (2016) Cytoskeletal Filamin A differentially modulates RNA polymerase III gene transcription in transformed cell lines. J Biol Chem 291:25239–25246
Li R, Chung AC, Dong Y, Yang W, Zhong X, Lan HY (2013) The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-beta/Smad3-Azin1 pathway. Kidney Int 84:1129–1144
Roelofs JJ, Rouschop KM, Leemans JC, Claessen N, de Boer AM, Frederiks WM, Lijnen HR, Weening JJ, Florquin S (2006) Tissue-type plasminogen activator modulates inflammatory responses and renal function in ischemia reperfusion injury. J Am Soc Nephrol JASN 17:131–140
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif.) 25:402–408
Tripathi V, Fei J, Ha T, Prasanth KV (2015) RNA fluorescence in situ hybridization in cultured mammalian cells. Methods Mol Biol 1206:123–136
Acknowledgements
This study was supported by the following Grants: Guangzhou Science, Technology and Innovation Commission (201806010123), Guangdong Basic and Applied Basic Research Foundation(2020A1515010247), Kelin Young Talents Program of the First Affiliated Hospital of Sun Yat-sen University (Y50179) National Key R&D Program of China (2016YFC0906101), Operational Grant of Guangdong Provincial Key Laboratory(2017B030314019), Guangdong Provincial Programme of Science and Technology(2017A050503003), Guangdong Provincial Programme of Science and Technology(2017B020227006), Guangzhou Municipal Programme of Science and Technology(201704020167), The Research Grants Council of Hong Kong (14163317, 14117418, 14104019, R4012-18F, and C7018-16G). The Health and Medical Research Fund of Hong Kong (HMRF 05161326, 14152321); The Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology Department (2019B121205005).
Author information
Authors and Affiliations
Contributions
HL and QZ designed the study and revised the manuscript. PZ and CY performed the experiments and wrote the manuscript. JY collected data and did the statistical analysis. ZL prepared figures. All authors have read and approved the final submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2020_3659_MOESM4_ESM.xlsx
Supplementary Table 4. Arid2-IR associated proteins or peptides identified by mass spectrometry in mTECs untreated or treated by IL-1β (10 ng/mL) for 30min (XLSX 30 kb)
18_2020_3659_MOESM5_ESM.xlsx
Supplementary Table 5. Top ten significant GO catalogs of potential Arid2-IR interacting proteins identified by mass spectrometry (XLSX 17 kb)
18_2020_3659_MOESM6_ESM.xlsx
Supplementary Table 6. Top fifteen significant KEGG pathways of potential Arid2-IR interacting proteins identified by mass spectrometry (XLSX 14 kb)
18_2020_3659_MOESM9_ESM.ab1
Supplementary File 2a. DNA sequencing results of pGL3-NLRC5-promoter. The inserted nucleotides were sequenced by RV3 primers. (AB1 271 kb)
18_2020_3659_MOESM10_ESM.ab1
Supplementary File 2b. DNA sequencing results of pGL3-NLRC5-promoter. The inserted nucleotides were sequenced by GLp2 primers (AB1 269 kb)

18_2020_3659_MOESM11_ESM.png
Supplementary Figure 1. QPCR was used to detect relative level of NLRC5 in Arid2-IR siRNA-1, siRNA-2, siRNA-3 or NC siRNA transfected mTECs. ***p < 0.001 versus NC siRNA, ##p < 0.01 Arid2-IR siRNA-1versus Arid2-IR siRNA-2.A si-1, 2, 3 were short for Arid2-IR siRNA-1, 2, 3 (PNG 7 kb)

18_2020_3659_MOESM12_ESM.png
Supplementary Figure 2. Statistical of Flna and NLRC5 protein level in UUO(A) and IRI(B) kidney respectively. * p < 0.05 versus control mice (PNG 344 kb)
18_2020_3659_MOESM13_ESM.pdf
Supplementary Figure 3. (A) Statistical of the protein level of phosho-IKK, IKKβ, phosho-p65, p65, phosho-IκB, IκB in IL-1β (10 ng/mL) treated mTECs at different time points. *p < 0.05, ***p < 0.001 versus 0h. (B)Statistical of NLRC5 protein level in NC or Arid2-IR siRNA-1 transfected mTECs. MTECs were then treated by IL-1β (10 ng/mL) at different time points. *p < 0.05, **p < 0.01, ***p < 0.001 versus 0h. (C) Statistical of NLRC5 protein level in NLRC5 or NC siRNA transfected mTECs. **p < 0.01 versus con; ###p < 0.001 versus NC. (D) Statistical of the protein level of phosho-IKK, IKKβ, phosho-p65, p65, phosho-IκB, IκB in IL-1β (10 ng/mL) treated mTECs at 0, 5m, 30m. **p < 0.01, ***p < 0.001 versus 0h; ##p < 0.01, ###p < 0.001 versus NC siRNA at same time point; &&p < 0.01, &&&p < 0.001 versus Arid2-IR siRNA-1 at same time point (PDF 160 kb)

18_2020_3659_MOESM14_ESM.png
Supplementary Figure 4. (A)Statistical of Flna protein level in NC or Flna siRNA treated mTECs. **p < 0.01 versus con; ##p < 0.01 versus NC. (B)The apoptosis rate of mTECs had no significantly change after transfecting with transfected with Arid2-IR siRNA-1 or NC siRNA (n = 3 for each group) (PNG 39 kb)

18_2020_3659_MOESM15_ESM.png
Supplementary Figure 5. Immunofluorescence of E-cadherin (green, left panel) and a-SMA (green, right panel) of mTECs, Over 95% cells were E-cadherin positive and a-SMA negative. DAPI staining is blue. Magnification: ×200 (PNG 379 kb)

18_2020_3659_MOESM16_ESM.png
Supplementary Figure 6. Pearson’ s correlation analysis was performed base on transcript abundance (RPKM) of the gene in Arid2-IR siRNA-1 (n=3) and NC (n=3) siRNA transfected groups (PNG 133 kb)
Rights and permissions
About this article
Cite this article
Zhang, P., Yu, C., Yu, J. et al. Arid2-IR promotes NF-κB-mediated renal inflammation by targeting NLRC5 transcription. Cell. Mol. Life Sci. 78, 2387–2404 (2021). https://doi.org/10.1007/s00018-020-03659-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03659-9