Abstract
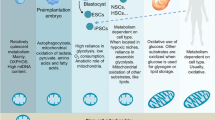
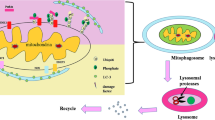
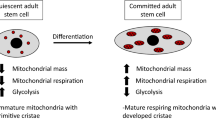
“Cellular reprogramming” facilitates the generation of desired cellular phenotype through the cell fate transition by affecting the mitochondrial dynamics and metabolic reshuffle in the embryonic and somatic stem cells. Interestingly, both the processes of differentiation and dedifferentiation witness a drastic and dynamic alteration in the morphology, number, distribution, and respiratory capacity of mitochondria, which are tightly regulated by the fission/fusion cycle, and mitochondrial clearance through autophagy following mitochondrial fission. Intriguingly, mitophagy is said to be essential in the differentiation of stem cells into various lineages such as erythrocytes, eye lenses, neurites, myotubes, and M1 macrophages. Mitophagy is also believed to play a central role in the dedifferentiation of a terminally differentiated cell into an induced pluripotent cell and in the acquisition of ‘stemness’ in cancer cells. Mitophagy-induced alteration in the mitochondrial dynamics facilitates metabolic shift, either into a glycolytic phenotype or into an OXPHOS phenotype, depending on the cellular demand. Mitophagy-induced rejuvenation of mitochondria regulates the transition of bioenergetics and metabolome, remodeling which facilitates an alteration in their cellular developmental capability. This review describes the detailed mechanism of the process of mitophagy and its association with cellular programming through alteration in the mitochondrial energetics. The metabolic shift post mitophagy is suggested to be a key factor in the cell fate transition during differentiation and dedifferentiation.





Similar content being viewed by others
References
Gurdon JB, Elsdale TR, Fischberg M (1958) Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature 182:64
Briggs R, King TJ (1952) Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc Natl Acad Sci 38:455–463
Wilmut I, Schnieke AE, Mcwhir J et al (2007) Viable offspring derived from fetal and adult mammalian cells. Cloning Stem Cell 9:3–7
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Wernig M, Meissner A, Cassady JP et al (2008) c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2:10–12
Brambrink T, Foreman R, Welstead GG et al (2008) Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2:151–159
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462:245–253
De Duve C, Wattiaux R (1966) Functions of lysosomes. Ann Rev Physiol 28:435–492
Nasrallah CM, Horvath TL (2014) Mitochondrial dynamics in the central regulation of metabolism. Nat Rev Endocrinol 10:650
Mattenberger Y, James DI, Martinou JC (2003) Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin. FEBS Lett 538:53–59
Twig G, Elorza A, Molina AJ et al (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433–446
Xu X, Duan S, Yi F et al (2013) Mitochondrial regulation in pluripotent stem cells. Cell Metab 18:325–332
Prigione A, Lichtner B, Kuhl H et al (2011) Human induced pluripotent stem cells harbor homoplasmic and heteroplasmic mitochondrial DNA mutations while maintaining human embryonic stem cell–like metabolic reprogramming. Stem Cell 9:1338–1348
Fang D, Yan S, Yu Q, Chen D et al (2016) Mfn2 is required for mitochondrial development and synapse formation in human induced pluripotent stem cells/hiPSC derived cortical neurons. Sci Rep 6:31462
Wanet A, Arnould T, Najimi M et al (2015) Connecting mitochondria, metabolism, and stem cell fate. Stem Cells Dev 24:1957–1971
Folmes CD, Nelson TJ, Martinez-Fernandez A et al (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab 14:264–271
Facucho-Oliveira JM, Alderson J, Spikings EC et al (2007) Mitochondrial DNA replication during differentiation of murine embryonic stem cells. J Cell Sci 120:4025–4034
Prigione A, Ruiz-Pérez MV, Bukowiecki R et al (2015) Metabolic restructuring and cell fate conversion. Cell Mol Life Sci 72:1759–1777
Bukowiecki R, Adjaye J, Prigione A (2014) Mitochondrial function in pluripotent stem cells and cellular reprogramming. Gerontology 60:174–182
Zhang J, Khvorostov I, Hong JS et al (2011) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 30:4860–4873
Varum S, Rodrigues AS, Moura MB et al (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6:e20914
Todd LR, Damin MN, Gomathinayagam R, Horn SR, Means AR, Sankar U (2010) Growth factor erv1-like modulates Drp1 to preserve mitochondrial dynamics and function in mouse embryonic stem cells. Mol Biol Cell 21:1225–1236
Youle RJ, Van Der Bliek AM (2012) Mitochondrial fission, fusion, and stress. Science 337:1062–1065
Prigione A, Fauler B, Lurz R et al (2010) The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cell 28:721–733
Armstrong L, Tilgner K, Saretzki G et al (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cell 28:661–673
Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8:774–785
Egan DF, Shackelford DB, Mihaylova MM et al (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461
Liang J, Xu ZX, Ding Z et al (2015) Myristoylation confers noncanonical AMPK functions in autophagy selectivity and mitochondrial surveillance. Nat Commun 6:7926
Birgisdottir ÅB, Lamark T, Johansen T (2013) The LIR motif–crucial for selective autophagy. J Cell Sci 126:3237–3247
Narendra D, Tanaka A, Suen DF et al (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183:795–803
Noda NN, Ohsumi Y, Inagaki F (2010) Atg8-family interacting motif crucial for selective autophagy. FEBS Lett 584:1379–1385
Noda NN, Kumeta H, Nakatogawa H et al (2008) Structural basis of target recognition by Atg8/LC3 during selective autophagy. Genes Cell 13:1211–1218
Pankiv S, Clausen TH, Lamark T et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Ichimura Y, Kumanomidou T, Sou YS et al (2008) Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem 283:22847–22857
Sarraf SA, Raman M, Guarani-Pereira V et al (2013) Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496:372
Lazarou M, Sliter DA, Kane LA et al (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309
Lamark T, Kirkin V, Dikic I et al (2009) NBR1 and p62 as cargo receptors for selective autophagy of ubiquitinated targets. Cell Cycle 8:1986–1990
Wong YC, Holzbaur EL (2014) Optineurin is an autophagy receptor for damaged mitochondria in Parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci 111:E4439–E4448
Novak I (2012) Mitophagy: a complex mechanism of mitochondrial removal. Antioxid Redox Signal 17:794–802
Zhang J, Ney PA (2009) Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 16:939–946
Zhu Y, Massen S, Terenzio M et al (2013) Modulation of serines 17 and 24 in the LC3-interacting region of Bnip3 determines pro-survival mitophagy versus apoptosis. J Biol Chem 288:1099–1113
Hanna RA, Quinsay MN, Orogo AM et al (2012) Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem 287:19094–19104
Novak I, Kirkin V, McEwan DG et al (2010) Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep 11:45–51
Liu L, Feng D, Chen G et al (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14:177
Wu W, Tian W, Hu Z et al (2014) ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep 15:566–575
Murakawa T, Yamaguchi O, Hashimoto A et al (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun 6:7527
Otsu K, Murakawa T, Yamaguchi O (2015) BCL2L13 is a mammalian homolog of the yeast mitophagy receptor Atg32. Autophagy 11:1932–1933
Sentelle RD, Senkal CE, Jiang W et al (2012) Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat Chem Biol 8:831–838
Chu CT, Ji J, Dagda RK et al (2013) Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol 15:1197
Jin SM, Lazarou M, Wang C et al (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191:933–942
Meissner C, Lorenz H, Weihofen A et al (2011) The mitochondrial intramembrane protease PARL cleaves human Pink1 to regulate Pink1 trafficking. J Neurochem 117:856–867
Narendra DP, Jin SM, Tanaka A et al (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8:e1000298
Matsuda N, Sato S, Shiba K et al (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189:211–221
Chen Y, Dorn GW (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340:471–475
Kane LA, Lazarou M, Fogel AI et al (2014) PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol 205:143–153
Koyano F, Okatsu K, Kosako H et al (2014) Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510:162
Ordureau A, Sarraf SA, Duda DM et al (2014) Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell 56:360–375
Chan NC, Salazar AM, Pham AH et al (2011) Broad activation of the ubiquitin–proteasome system by Parkin is critical for mitophagy. Hum Mol Genet 20:1726–1737
Lim KL, Dawson VL, Dawson TM (2006) Parkin-mediated lysine 63-linked polyubiquitination: a link to protein inclusions formation in Parkinson’s and other conformational diseases? Neurobiol Aging 27:524–529
Geisler S, Holmström KM, Skujat D et al (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12:119
Wei Y, Chiang WC, Sumpter R et al (2017) Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168(224–38):e10
Hollville E, Carroll RG, Cullen SP et al (2014) Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol Cell 55:451–466
Narendra D, Kane LA, Hauser DN et al (2010) p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 6:1090–1106
Michiorri S, Gelmetti V, Giarda E et al (2010) The Parkinson-associated protein PINK1 interacts with beclin1 and promotes autophagy. Cell Death Differ 17:962
Van Humbeeck C, Cornelissen T, Hofkens H et al (2011) Parkin interacts with Ambra1 to induce mitophagy. J Neurosci 31:10249–10261
Strappazzon F, Vietri-Rudan M, Campello S et al (2011) Mitochondrial BCL-2 inhibits AMBRA1-induced autophagy. EMBO J 30:1195–1208
Strappazzon F, Nazio F, Corrado M et al (2015) AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ 22:419
Orvedahl A, Sumpter R Jr, Xiao G et al (2011) Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 480:113
Park J, Lee SB, Lee S et al (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441:1157–1161
Sandoval H, Thiagarajan P, Dasgupta SK et al (2008) Essential role for Nix in autophagic maturation of erythroid cells. Nature 454:232
Yamaguchi O, Murakawa T, Nishida K et al (2016) Receptor-mediated mitophagy. J Mol Cell Cardiol 95:50–56
Hamacher-Brady A, Brady N, Logue S et al (2007) Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 14:146
Hamacher-Brady A, Brady NR (2016) Mitophagy programs: mechanisms and physiological implications of mitochondrial targeting by autophagy. Cell Mol Life Sci 73:775–795
Melser S, Chatelain EH, Lavie J et al (2013) Rheb regulates mitophagy induced by mitochondrial energetic status. Cell Metab 17:719–730
Chen G, Han Z, Feng D et al (2014) A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell 54:362–377
Wu H, Xue D, Chen G et al (2014) The BCL2L1 and PGAM5 axis defines hypoxia-induced receptor-mediated mitophagy. Autophagy 10:1712–1725
Bian Y, Song C, Cheng K et al (2014) An enzyme assisted RP-RPLC approach for in-depth analysis of human liver phosphoproteome. J Proteom 96:253–262
Panda PK, Naik PP, Meher BR et al (2018) PUMA dependent mitophagy by Abrus agglutinin contributes to apoptosis through ceramide generation. Biochim Biophys Acta Mol Cell Res 1865:480–495
McLelland GL, Soubannier V, Chen CX et al (2014) Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33:282–295
Miyamoto Y, Kitamura N, Nakamura Y et al (2011) Possible existence of lysosome-like organella within mitochondria and its role in mitochondrial quality control. PLoS One 6:e16054
Hamacher-Brady A, Choe S, Krijnse-Locker J et al (2014) Intramitochondrial recruitment of endolysosomes mediates Smac degradation and constitutes a novel intrinsic apoptosis antagonizing function of XIAP E3 ligase. Cell Death Differ 21:1862
Hamacher-Brady A, Brady NR (2015) Bax/Bak-dependent, Drp1-independent targeting of XIAP into inner-mitochondrial compartments counteracts Smac-dependent effector caspase activation. J Biol Chem M115:643064
Kitamura N, Nakamura Y, Miyamoto Y et al (2011) Mieap, a p53-inducible protein, controls mitochondrial quality by repairing or eliminating unhealthy mitochondria. PLoS One 6:e16060
Begus-Nahrmann Y, Lechel A, Obenauf AC et al (2009) p53 deletion impairs clearance of chromosomal-instable stem cells in aging telomere-dysfunctional mice. Nat Genet 41:1138
Liu K, Lee J, Kim JY et al (2017) Mitophagy controls the activities of tumor suppressor p53 to regulate hepatic cancer stem cells. Mol Cell 68(281–92):e5
Koehler CL, Perkins GA, Ellisman MH et al (2017) Pink1 and Parkin regulate Drosophila intestinal stem cell proliferation during stress and aging. J Cell Biol 216:2315–2327
Mahrouf-Yorgov M, Augeul L, Da Silva CC et al (2017) Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ 24:1224
Phinney DG, Di Giuseppe M, Njah J et al (2015) Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun 6:8472
Mortensen M, Soilleux EJ, Djordjevic G et al (2011) The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med 208:455–467
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4:487–492
Theunissen TW, Powell BE, Wang H et al (2014) Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15:471–487
Takashima Y, Guo G, Loos R et al (2014) Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158:1254–1269
Teslaa T, Teitell M (2015) Pluripotent stem cell energy metabolism: an update. EMBO J 34:138–153
Vazquez-Martin A, Van den Haute C, Cufí S et al (2016) Mitophagy-driven mitochondrial rejuvenation regulates stem cell fate. Aging 8:1330
Bordt EA, Clerc P, Roelofs BA et al (2017) The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell 40:583–594
Vazquez-Martin A, Cufí S, Corominas-Faja B et al (2012) Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging 4:393
Xiang G, Yang L, Long Q et al (2017) BNIP3L-dependent mitophagy accounts for mitochondrial clearance during 3 factors-induced somatic cell reprogramming. Autophagy 13:1543–1555
Liu K, Zhao Q, Liu P et al (2016) ATG3-dependent autophagy mediates mitochondrial homeostasis in pluripotency acquirement and maintenance. Autophagy 12:2000–2008
Ito K, Turcotte R, Cui J et al (2016) Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science 354:1156–1160
Angelova PR, Barilani M, Lovejoy C et al (2017) Mitochondrial dysfunction in parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol 14:474–484
Marycz K, Kornicka K, Grzesiak J et al (2016) Macroautophagy and selective mitophagy ameliorate chondrogenic differentiation potential in adipose stem cells of equine metabolic syndrome: new findings in the field of progenitor cells differentiation. Oxid Med Cell Longev 2017:3861790
Song M, Mihara K, Chen Y et al (2015) Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 21:273–285
Mao K, Klionsky DJ (2013) Mitochondrial fission facilitates mitophagy in Saccharomyces cerevisiae. Autophagy 9:1900–1901
Frank M, Duvezin-Caubet S, Koob S et al (2012) Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim et Biophys Acta (BBA) Mol Cell Res 1823:2297–2310
Chen H, Chan DC (2009) Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum Mol Genet 18:R169–R176
Son MY, Choi H, Han YM et al (2013) Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem Cells 31:2374–2387
Todd LR, Gomathinayagam R, Sankar U (2010) A novel Gfer-Drp1 link in preserving mitochondrial dynamics and function in pluripotent stem cells. Autophagy 6:821–822
Prieto J, León M, Ponsoda X et al (2016) Dysfunctional mitochondrial fission impairs cell reprogramming. Cell Cycle 15:3240–3250
Wang L, Zhang T, Wang L et al (2017) Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. EMBO J 36:1330–1347
Son M, Kwon Y, Son M et al (2015) Mitofusins deficiency elicits mitochondrial metabolic reprogramming to pluripotency. Cell Death Differ 22:1957–1969
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105
Naik PP, Das DN, Panda PK et al (2016) Implications of cancer stem cells in developing therapeutic resistance in oral cancer. Oral Oncol 62:122–135
Naik PP, Mukhopadhyay S, Panda PK et al (2018) Autophagy regulates cisplatin-induced stemness and chemoresistance via the upregulation of CD44, ABCB1 and ADAM17 in oral squamous cell carcinoma. Cell Prolif 51:e12411
Zhou TJ, Zhang SL, He CY et al (2017) Downregulation of mitochondrial cyclooxygenase-2 inhibits the stemness of nasopharyngeal carcinoma by decreasing the activity of dynamin-related protein 1. Theranostics 7:1389
Shen YA, Wang CY, Hsieh YT et al (2015) Metabolic reprogramming orchestrates cancer stem cell properties in nasopharyngeal carcinoma. Cell Cycle 14:86–98
Whelan KA, Chandramouleeswaran PM, Tanaka K et al (2017) Autophagy supports generation of cells with high CD44 expression via modulation of oxidative stress and Parkin-mediated mitochondrial clearance. Oncogene 36:4843–4858
Yan C, Luo L, Guo CY et al (2017) Doxorubicin-induced mitophagy contributes to drug resistance in cancer stem cells from HCT8 human colorectal cancer cells. Cancer Lett 388:34–42
Barde I, Rauwel B, Marin-Florez RM et al (2013) A KRAB/KAP1-miRNA cascade regulates erythropoiesis through stage-specific control of mitophagy. Science 340:350–353
Wu L, Xu W, Xu L et al (2017) Mitophagy is increased during erythroid differentiation in β-thalassemia. Int J Hematol 105:162–173
Sin J, Andres AM, Taylor DJ et al (2016) Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 12:369–380
Kim B, Kim JS, Yoon Y et al (2013) Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am J Physiol Regul Integr Comp Physiol 305:R927–R938
Gong G, Song M, Csordas G, Kelly DP et al (2015) Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 350:aad2459
Xavier JM, Morgado AL, Sola S et al (2014) Mitochondrial translocation of p53 modulates neuronal fate by preventing differentiation-induced mitochondrial stress. Antioxid Redox Signal 21:1009–1024
Esteban-Martínez L, Sierra-Filardi E, McGreal RS et al (2017) Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J 36:1688–1706
Esteban-Martinez L, Boya P (2017) BNIP3L/NIX-dependent mitophagy regulates cell differentiation via metabolic reprogramming. Autophagy 14:915–917
Chauss D, Basu S, Rajakaruna S et al (2014) Differentiation state-specific mitochondrial dynamic regulatory networks are revealed by global transcriptional analysis of the developing chicken lens. G3 Genes Genomes Genet 4:1515–1527
Costello MJ, Brennan LA, Basu S et al (2013) Autophagy and mitophagy participate in ocular lens organelle degradation. Exp Eye Res 116:141–150
Larson-Casey JL, Deshane JS, Ryan AJ et al (2016) Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44:582–596
Goldman SJ, Zhang Y, Jin S (2011) Autophagic degradation of mitochondria in white adipose tissue differentiation. Antioxid Redox Signal 14:1971–1978
Altshuler-Keylin S, Shinoda K, Hasegawa Y et al (2016) Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab 24:402–419
Zhou W, Choi M, Margineantu D et al (2012) HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J 31:2103–2116
Corominas-Faja B, Cuyàs E, Gumuzio J et al (2014) Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget 5:8306
Sánchez-Cenizo L, Formentini L, Aldea M et al (2010) Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J Biol Chem 285:25308–25313
Willers IM, Cuezva JM (2011) Post-transcriptional regulation of the mitochondrial H+-ATP synthase: a key regulator of the metabolic phenotype in cancer. Biochim Biophys Acta (BBA) Bioenerget 1807:543–551
Sánchez-Aragó M, García-Bermúdez J, Martínez-Reyes I et al (2013) Degradation of IF1 controls energy metabolism during osteogenic differentiation of stem cells. EMBO Rep 14:638–644
Wenz T, Rossi SG, Rotundo RL et al (2009) Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci 106:20405–20410
Gerhart-Hines Z, Rodgers JT, Bare O et al (2007) Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J 26:1913–1923
Ryall JG, Dell’Orso S, Derfoul A et al (2015) The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 16:171–183
Panopoulos AD, Yanes O, Ruiz S et al (2012) The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res 22:168
Vazquez-Martin A, Corominas-Faja B, Cufi S et al (2013) The mitochondrial H+-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle 12:207–218
Acknowledgements
Research support was partly provided by Department of Biotechnology [Grant number: BT/PR7791/BRB/10/1187/2013; Science and Technology Department, Government of Odisha; the Board of Research in Nuclear Sciences (BRNS) [number: 37(1)/14/38/2016-BRNS/37276], Department of Atomic Energy (DAE); Science and Engineering Research Board (SERB) [number: EMR/2016/001246], Department of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Naik, P.P., Birbrair, A. & Bhutia, S.K. Mitophagy-driven metabolic switch reprograms stem cell fate. Cell. Mol. Life Sci. 76, 27–43 (2019). https://doi.org/10.1007/s00018-018-2922-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2922-9