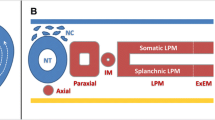
Abstract
Mesenchymoangioblast (MB) is the earliest precursor for endothelial and mesenchymal cells originating from APLNR+PDGFRα+KDR+ mesoderm in human pluripotent stem cell cultures. MBs are identified based on their capacity to form FGF2-dependent compact spheroid colonies in a serum-free semisolid medium. MBs colonies are composed of PDGFRβ+CD271+EMCN+DLK1+CD73− primitive mesenchymal cells which are generated through endothelial/angioblastic intermediates (cores) formed during first 3–4 days of clonogenic cultures. MB-derived primitive mesenchymal cells have potential to differentiate into mesenchymal stromal/stem cells (MSCs), pericytes, and smooth muscle cells. In this review, we summarize the specification and developmental potential of MBs, emphasize features that distinguish MBs from other mesenchymal progenitors described in the literature and discuss the value of these findings for identifying molecular pathways leading to MSC and vasculogenic cell specification, and developing cellular therapies using MB-derived progeny.



Similar content being viewed by others
Abbreviations
- MB:
-
Mesenchymoangioblast
- HB:
-
Hemangioblast
- PC:
-
Pericytes
- SMC:
-
Smooth muscle cells
- MSC:
-
Mesenchymal stem/stromal cells
- hPSC:
-
Human pluripotent stem cells
- hESC:
-
Human embryonic stem cells
- hiPSCs:
-
Human-induced pluripotent stem cells
References
Vodyanik MA, Yu J, Zhang X, Tian S, Stewart R, Thomson JA, Slukvin II (2010) A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7(6):718–729. https://doi.org/10.1016/j.stem.2010.11.011
Tam PP, Behringer RR (1997) Mouse gastrulation: the formation of a mammalian body plan. Mech Dev 68(1–2):3–25
Lawson A, Schoenwolf GC (2001) Cell populations and morphogenetic movements underlying formation of the avian primitive streak and organizer. Genesis 29(4):188–195
Sadler TW (2006) Langman’s medical embryology. Lippincott Williams and Wilkins, Philadelphia
Hay ED (2005) The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn 233(3):706–720. https://doi.org/10.1002/dvdy.20345
Noden DM (1978) The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol 67(2):296–312
Noden DM (1986) Origins and patterning of craniofacial mesenchymal tissues. J Craniofac Genet Dev Biol Suppl 2:15–31
Mayor R, Theveneau E (2013) The neural crest. Development 140(11):2247–2251. https://doi.org/10.1242/dev.091751
Majesky MW (2007) Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27(6):1248–1258. https://doi.org/10.1161/ATVBAHA.107.141069
Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S (2007) Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129(7):1377–1388. https://doi.org/10.1016/j.cell.2007.04.028
Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S (2014) The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3:e03696. https://doi.org/10.7554/eLife.03696
Miller FD (2007) Riding the waves: neural and nonneural origins for mesenchymal stem cells. Cell Stem Cell 1(2):129–130. https://doi.org/10.1016/j.stem.2007.07.007
Morikawa S, Mabuchi Y, Niibe K, Suzuki S, Nagoshi N, Sunabori T, Shimmura S, Nagai Y, Nakagawa T, Okano H, Matsuzaki Y (2009) Development of mesenchymal stem cells partially originate from the neural crest. Biochem Biophys Res Commun 379(4):1114–1119. https://doi.org/10.1016/j.bbrc.2009.01.031
Rossant J, Tam PPL (2017) New insights into early human development: lessons for stem cell derivation and differentiation. Cell Stem Cell 20(1):18–28. https://doi.org/10.1016/j.stem.2016.12.004
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920. https://doi.org/10.1126/science.1151526
Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF, Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MH, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgard PO, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA (2015) Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol 17(8):994–1003. https://doi.org/10.1038/ncb3205
Orlova VV, Drabsch Y, Freund C, Petrus-Reurer S, van den Hil FE, Muenthaisong S, Dijke PT, Mummery CL (2014) Functionality of endothelial cells and pericytes from human pluripotent stem cells demonstrated in cultured vascular plexus and zebrafish xenografts. Arterioscler Thromb Vasc Biol 34(1):177–186. https://doi.org/10.1161/ATVBAHA.113.302598
Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, Germanguz I, Amit M, Itskovitz-Eldor J (2012) Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation 125(1):87–99. https://doi.org/10.1161/CIRCULATIONAHA.111.048264
Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S (2012) Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol 30(2):165–173. https://doi.org/10.1038/nbt.2107
Uenishi G, Theisen D, Lee JH, Kumar A, Raymond M, Vodyanik M, Swanson S, Stewart R, Thomson J, Slukvin I (2014) Tenascin C promotes hematoendothelial development and T lymphoid commitment from human pluripotent stem cells in chemically defined conditions. Stem Cell Rep 3(6):1073–1084. https://doi.org/10.1016/j.stemcr.2014.09.014
Flamme I, Breier G, Risau W (1995) Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol 169(2):699–712. https://doi.org/10.1006/dbio.1995.1180
Risau W, Flamme I (1995) Vasculogenesis. Annu Rev Cell Dev Biol 11:73–91. https://doi.org/10.1146/annurev.cb.11.110195.000445
Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II (2017) Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep 19(9):1902–1916. https://doi.org/10.1016/j.celrep.2017.05.019
Hafner AL, Contet J, Ravaud C, Yao X, Villageois P, Suknuntha K, Annab K, Peraldi P, Binetruy B, Slukvin II, Ladoux A, Dani C (2016) Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Sci Rep 6:32490. https://doi.org/10.1038/srep32490
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21(2):193–215. https://doi.org/10.1016/j.devcel.2011.07.001
Shen EM, McCloskey KE (2017) Development of mural cells: from in vivo understanding to in vitro recapitulation. Stem Cells Dev 26(14):1020–1041. https://doi.org/10.1089/scd.2017.0020
D’Aniello C, Lonardo E, Iaconis S, Guardiola O, Liguoro AM, Liguori GL, Autiero M, Carmeliet P, Minchiotti G (2009) G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circ Res 105(3):231–238. https://doi.org/10.1161/CIRCRESAHA.109.201186
Devic E, Paquereau L, Vernier P, Knibiehler B, Audigier Y (1996) Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev 59(2):129–140
Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L (2007) Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell 12(3):391–402. https://doi.org/10.1016/j.devcel.2007.01.011
Orr-Urtreger A, Bedford MT, Do MS, Eisenbach L, Lonai P (1992) Developmental expression of the alpha receptor for platelet-derived growth factor, which is deleted in the embryonic lethal Patch mutation. Development 115(1):289–303
Sakurai H, Era T, Jakt LM, Okada M, Nakai S, Nishikawa S (2006) In vitro modeling of paraxial and lateral mesoderm differentiation reveals early reversibility. Stem Cells 24(3):575–586. https://doi.org/10.1634/stemcells.2005-0256
Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J (1993) flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118(2):489–498
Choi KD, Vodyanik MA, Togarrati PP, Suknuntha K, Kumar A, Samarjeet F, Probasco MD, Tian S, Stewart R, Thomson JA, Slukvin II (2012) Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep 2(3):553–567. https://doi.org/10.1016/j.celrep.2012.08.002
Slukvin II (2013) Deciphering the hierarchy of angiohematopoietic progenitors from human pluripotent stem cells. Cell Cycle 12(5):720–727. https://doi.org/10.4161/cc.23823
Flamme I, Frolich T, Risau W (1997) Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol 173(2):206–210. https://doi.org/10.1002/(SICI)1097-4652(199711)173:2<206:AID-JCP22>3.0.CO;2-C
Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495(7440):227–230. https://doi.org/10.1038/nature11926
Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS (2015) Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. https://doi.org/10.1126/science.aad0084
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313. https://doi.org/10.1016/j.stem.2008.07.003
Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G (2002) The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 129(11):2773–2783
Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M (2007) Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol Chapter 2:Unit 2B 1. https://doi.org/10.1002/9780470151808.sc02b01s3
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G (2007) Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9(3):255–267. https://doi.org/10.1038/ncb1542
Loperfido M, Jarmin S, Dastidar S, Di Matteo M, Perini I, Moore M, Nair N, Samara-Kuko E, Athanasopoulos T, Tedesco FS, Dickson G, Sampaolesi M, VandenDriessche T, Chuah MK (2016) piggyBac transposons expressing full-length human dystrophin enable genetic correction of dystrophic mesoangioblasts. Nucleic Acids Res 44(2):744–760. https://doi.org/10.1093/nar/gkv1464
Iyer PS, Mavoungou LO, Ronzoni F, Zemla J, Schmid-Siegert E, Antonini S, Neff LA, Dorchies OM, Jaconi M, Lekka M, Messina G, Mermod N (2018) Autologous cell therapy approach for duchenne muscular dystrophy using piggybac transposons and mesoangioblasts. Mol Ther 26(4):1093–1108. https://doi.org/10.1016/j.ymthe.2018.01.021
Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E, Tonlorenzi R, Ragazzi M, Calderazzi G, Hoshiya H, Cappellari O, Mora M, Schoser B, Schneiderat P, Oshimura M, Bottinelli R, Sampaolesi M, Torrente Y, Broccoli V, Cossu G (2012) Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med 4(140):140ra189. https://doi.org/10.1126/scitranslmed.3003541
Dekel I, Magal Y, Pearson-White S, Emerson CP, Shani M (1992) Conditional conversion of ES cells to skeletal muscle by an exogenous MyoD1 gene. New Biol 4(3):217–224
Rao L, Tang W, Wei Y, Bao L, Chen J, Chen H, He L, Lu P, Ren J, Wu L, Luan Z, Cui C, Xiao L (2012) Highly efficient derivation of skeletal myotubes from human embryonic stem cells. Stem Cell Rev 8(4):1109–1119. https://doi.org/10.1007/s12015-012-9413-4
Davis RL, Weintraub H, Lassar AB (1987) Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51(6):987–1000
Tanaka A, Woltjen K, Miyake K, Hotta A, Ikeya M, Yamamoto T, Nishino T, Shoji E, Sehara-Fujisawa A, Manabe Y, Fujii N, Hanaoka K, Era T, Yamashita S, Isobe K, Kimura E, Sakurai H (2013) Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro. PLoS One 8(4):e61540. https://doi.org/10.1371/journal.pone.0061540
Bonfanti C, Rossi G, Tedesco FS, Giannotta M, Benedetti S, Tonlorenzi R, Antonini S, Marazzi G, Dejana E, Sassoon D, Cossu G, Messina G (2015) PW1/Peg3 expression regulates key properties that determine mesoangioblast stem cell competence. Nat Commun 6:6364. https://doi.org/10.1038/ncomms7364
Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G (2004) Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432(7017):625–630. https://doi.org/10.1038/nature03122
Kattman SJ, Huber TL, Keller GM (2006) Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11(5):723–732. https://doi.org/10.1016/j.devcel.2006.10.002
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM (2008) Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453(7194):524–528. https://doi.org/10.1038/nature06894
Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G (1997) A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386(6624):488–493. https://doi.org/10.1038/386488a0
Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G (1998) A common precursor for hematopoietic and endothelial cells. Development 125(4):725–732
Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G (2009) The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457(7231):892–895. https://doi.org/10.1038/nature07679
Kimbrel EA, Kouris NA, Yavanian GJ, Chu J, Qin Y, Chan A, Singh RP, McCurdy D, Gordon L, Levinson RD, Lanza R (2014) Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev 23(14):1611–1624. https://doi.org/10.1089/scd.2013.0554
Gertow K, Hirst CE, Yu QC, Ng ES, Pereira LA, Davis RP, Stanley EG, Elefanty AG (2013) WNT3A promotes hematopoietic or mesenchymal differentiation from hESCs depending on the time of exposure. Stem Cell Rep 1(1):53–65. https://doi.org/10.1016/j.stemcr.2013.04.002
Faloon P, Arentson E, Kazarov A, Deng CX, Porcher C, Orkin S, Choi K (2000) Basic fibroblast growth factor positively regulates hematopoietic development. Development 127(9):1931–1941
Robertson SM, Kennedy M, Shannon JM, Keller G (2000) A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development 127(11):2447–2459
Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G (2011) Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8(2):228–240. https://doi.org/10.1016/j.stem.2010.12.008
Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G (2007) Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood 109(7):2679–2687. https://doi.org/10.1182/blood-2006-09-047704
Slukvin II, Vodyanik M (2011) Endothelial origin of mesenchymal stem cells. Cell Cycle 10(9):1370–1373
Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K (2000) Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408(6808):92–96. https://doi.org/10.1038/35040568
Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, Scholes J, Dravid G, Li X, MacLellan WR, Crooks GM (2010) Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci USA 107(31):13742–13747. https://doi.org/10.1073/pnas.1002077107
Chin CJ, Cooper AR, Lill GR, Evseenko D, Zhu Y, He CB, Casero D, Pellegrini M, Kohn DB, Crooks GM (2016) Genetic tagging during human mesoderm differentiation reveals tripotent lateral plate mesodermal progenitors. Stem Cells 34(5):1239–1250. https://doi.org/10.1002/stem.2351
Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Peault B (2014) Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci 71(8):1353–1374. https://doi.org/10.1007/s00018-013-1462-6
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54. https://doi.org/10.1038/emm.2013.94
Sharma RR, Pollock K, Hubel A, McKenna D (2014) Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 54(5):1418–1437. https://doi.org/10.1111/trf.12421
Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F (2017) Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med 9(416):eaam7828. https://doi.org/10.1126/scitranslmed.aam7828
Majka M, Sulkowski M, Badyra B, Musialek P (2017) Concise review: mesenchymal stem cells in cardiovascular regeneration: emerging research directions and clinical applications. Stem Cells Transl Med 6(10):1859–1867. https://doi.org/10.1002/sctm.16-0484
Munneke JM, Spruit MJA, Cornelissen AS, van Hoeven V, Voermans C, Hazenberg MD (2016) The potential of mesenchymal stromal cells as treatment for severe steroid-refractory acute graft-versus-host disease: a critical review of the literature. Transplantation 100(11):2309–2314
Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR (2014) MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14(2):141–145. https://doi.org/10.1016/j.stem.2014.01.013
Viswanathan S, Keating A, Deans R, Hematti P, Prockop D, Stroncek DF, Stacey G, Weiss DJ, Mason C, Rao MS (2014) Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev 23(11):1157–1167. https://doi.org/10.1089/scd.2013.0591
Martin I, De Boer J, Sensebe L, Therapy MSCCotISfC (2016) A relativity concept in mesenchymal stromal cell manufacturing. Cytotherapy 18(5):613–620. https://doi.org/10.1016/j.jcyt.2016.02.004
Liu ST, de Castro LF, Jin P, Civini S, Ren JQ, Reems JA, Cancelas J, Nayak R, Shaw G, O’Brien T, McKenna DH, Armant M, Silberstein L, Gee AP, Hei DJ, Hematti P, Kuznetsov SA, Robey PG, Stroncek DF (2017) Manufacturing differences affect human bone marrow stromal cell characteristics and function: comparison of production methods and products from multiple centers. Sci Rep 7:46731
Chin CJ, Li S, Corselli M, Casero D, Zhu Y, He CB, Hardy R, Peault B, Crooks GM (2018) Transcriptionally and functionally distinct mesenchymal subpopulations are generated from human pluripotent stem cells. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2017.12.005
Luzzani CD, Miriuka SG (2017) Pluripotent stem cells as a robust source of mesenchymal stem cells. Stem Cell Rev 13(1):68–78. https://doi.org/10.1007/s12015-016-9695-z
Xu J, Gong T, Heng BC, Zhang CF (2017) A systematic review: differentiation of stem cells into functional pericytes. FASEB J 31(5):1775–1786. https://doi.org/10.1096/fj.201600951RRR
Maguire EM, Xiao Q, Xu Q (2017) Differentiation and application of induced pluripotent stem cell-derived vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 37(11):2026–2037. https://doi.org/10.1161/ATVBAHA.117.309196
Neofytou E, O’Brien CG, Couture LA, Wu JC (2015) Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Investig 125(7):2551–2557. https://doi.org/10.1172/JCI80575
Trounson A, DeWitt ND (2016) Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 17(3):194–200. https://doi.org/10.1038/nrm.2016.10
Tapia A, Salgado MS, Martin MP, Lapuerta M, Rodriguez-Fernandez J, Rossi MJ, Cabanas B (2016) Molecular characterization of the gas-particle interface of soot sampled from a diesel engine using a titration method. Environ Sci Technol 50(6):2946–2955. https://doi.org/10.1021/acs.est.5b05531
Takahashi K, Yamanaka S (2016) A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol 17(3):183–193. https://doi.org/10.1038/nrm.2016.8
Abyzov A, Mariani J, Palejev D, Zhang Y, Haney MS, Tomasini L, Ferrandino AF, Rosenberg Belmaker LA, Szekely A, Wilson M, Kocabas A, Calixto NE, Grigorenko EL, Huttner A, Chawarska K, Weissman S, Urban AE, Gerstein M, Vaccarino FM (2012) Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492(7429):438–442. https://doi.org/10.1038/nature11629
Cheng L, Hansen NF, Zhao L, Du Y, Zou C, Donovan FX, Chou BK, Zhou G, Li S, Dowey SN, Ye Z, Program NCS, Chandrasekharappa SC, Yang H, Mullikin JC, Liu PP (2012) Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell 10(3):337–344. https://doi.org/10.1016/j.stem.2012.01.005
Young MA, Larson DE, Sun CW, George DR, Ding L, Miller CA, Lin L, Pawlik KM, Chen K, Fan X, Schmidt H, Kalicki-Veizer J, Cook LL, Swift GW, Demeter RT, Wendl MC, Sands MS, Mardis ER, Wilson RK, Townes TM, Ley TJ (2012) Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell 10(5):570–582. https://doi.org/10.1016/j.stem.2012.03.002
Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E, Ng S, Sourour M, Hamalainen R, Olsson C, Lundin K, Mikkola M, Trokovic R, Peitz M, Brustle O, Bazett-Jones DP, Alitalo K, Lahesmaa R, Nagy A, Otonkoski T (2011) Copy number variation and selection during reprogramming to pluripotency. Nature 471(7336):58–62. https://doi.org/10.1038/nature09871
Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K (2011) Somatic coding mutations in human induced pluripotent stem cells. Nature 471(7336):63–67. https://doi.org/10.1038/nature09805
Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R, Lynch C, Harness JV, Lee S, Barrero MJ, Ku S, Martynova M, Semechkin R, Galat V, Gottesfeld J, Izpisua Belmonte JC, Murry C, Keirstead HS, Park HS, Schmidt U, Laslett AL, Muller FJ, Nievergelt CM, Shamir R, Loring JF (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8(1):106–118. https://doi.org/10.1016/j.stem.2010.12.003
Varela C, Denis JA, Polentes J, Feyeux M, Aubert S, Champon B, Pietu G, Peschanski M, Lefort N (2012) Recurrent genomic instability of chromosome 1q in neural derivatives of human embryonic stem cells. J Clin Investig 122(2):569–574. https://doi.org/10.1172/JCI46268
Lei Y, Schaffer DV (2013) A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci USA 110(52):E5039–E5048. https://doi.org/10.1073/pnas.1309408110
Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I (2004) Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22(5):675–682. https://doi.org/10.1634/stemcells.22-5-675
Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B (2006) Aging of mesenchymal stem cell in vitro. BMC Cell Biol 7:14. https://doi.org/10.1186/1471-2121-7-14
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA (2011) Chemically defined conditions for human iPSC derivation and culture. Nat Methods 8(5):424–429. https://doi.org/10.1038/nmeth.1593
Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, Xu RH (2014) Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Rep 3(1):115–130. https://doi.org/10.1016/j.stemcr.2014.04.020
Zhang Y, Liao S, Yang M, Liang X, Poon MW, Wong CY, Wang J, Zhou Z, Cheong SK, Lee CN, Tse HF, Lian Q (2012) Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transpl 21(10):2225–2239. https://doi.org/10.3727/096368912X653020
Hao Q, Zhu YG, Monsel A, Gennai S, Lee T, Xu F, Lee JW (2015) Study of bone marrow and embryonic stem cell-derived human mesenchymal stem cells for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells Transl Med 4(7):832–840. https://doi.org/10.5966/sctm.2015-0006
Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai YH, He J, Tse HF, Lian Q (2015) Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep 5:11235. https://doi.org/10.1038/srep11235
Koch JM, D’Souza SS, Schwahn DJ, Dixon I, Hacker TA (2016) Mesenchymoangioblast-derived mesenchymal stromal cells inhibit cell damage, tissue damage and improve peripheral blood flow following hindlimb ischemic injury in mice. Cytotherapy 18(2):219–228. https://doi.org/10.1016/j.jcyt.2015.10.013
Royce SG, Rele S, Broughton BRS, Kelly K, Samuel CS (2017) Intranasal administration of mesenchymoangioblast-derived mesenchymal stem cells abrogates airway fibrosis and airway hyperresponsiveness associated with chronic allergic airways disease. FASEB J 31(9):4168–4178. https://doi.org/10.1096/fj.201700178R
Acknowledgements
We thank Matthew Raymond for editorial assistance. I.I.S. and A.K are supported by funds from the National Institute of Health (U01HL134655, U01HL099773 and P51 RR000167). I.I.S. is a founding shareholder and consultant for Cynata Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slukvin, I.I., Kumar, A. The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell. Mol. Life Sci. 75, 3507–3520 (2018). https://doi.org/10.1007/s00018-018-2871-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2871-3