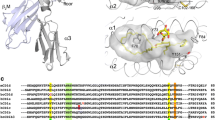
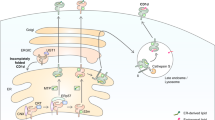
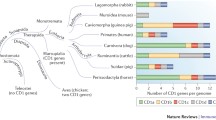
Abstract
The immune system has evolved to protect hosts from pathogens. T cells represent a critical component of the immune system by their engagement in host defence mechanisms against microbial infections. Our knowledge of the molecular recognition by T cells of pathogen-derived peptidic antigens that are presented by the major histocompatibility complex glycoproteins is now well established. However, lipids represent an additional, distinct chemical class of molecules that when presented by the family of CD1 antigen-presenting molecules can serve as antigens, and be recognized by specialized subsets of T cells leading to antigen-specific activation. Over the past decades, numerous CD1-presented self- and bacterial lipid-based antigens have been isolated and characterized. However, our understanding at the molecular level of T cell immunity to CD1 molecules presenting microbial lipid-based antigens is still largely unexplored. Here, we review the insights and the molecular basis underpinning the recognition of microbial lipid-based antigens by T cells.






Similar content being viewed by others
References
Miles JJ, McCluskey J, Rossjohn J, Gras S (2015) Understanding the complexity and malleability of T-cell recognition. Immunol Cell Biol 93(5):433–441. https://doi.org/10.1038/icb.2014.112
Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, McCluskey J (2015) T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol 33:169–200. https://doi.org/10.1146/annurev-immunol-032414-112334
Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB (1994) Recognition of a lipid antigen by CD1-restricted alpha beta + T cells. Nature 372(6507):691–694. https://doi.org/10.1038/372691a0
Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O’Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491(7426):717–723. https://doi.org/10.1038/nature11605
Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, Rossjohn J (2013) Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nature Commun 4:2142. https://doi.org/10.1038/ncomms3142
Adams EJ, Luoma AM (2013) The adaptable major histocompatibility complex (MHC) fold: structure and function of nonclassical and MHC class I-like molecules. Annu Rev Immunol 31:529–561. https://doi.org/10.1146/annurev-immunol-032712-095912
Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB (2015) Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol 15(10):643–654. https://doi.org/10.1038/nri3889
Zajonc DM, Flajnik MF (2016) CD1, MR1, NKT, and MAIT: evolution and origins of non-peptidic antigen recognition by T lymphocytes. Immunogenetics 68(8):489–490. https://doi.org/10.1007/s00251-016-0941-y
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB (2015) The burgeoning family of unconventional T cells. Nat Immunol 16(11):1114–1123. https://doi.org/10.1038/ni.3298
Garcia-Alles LF, Giacometti G, Versluis C, Maveyraud L, de Paepe D, Guiard J, Tranier S, Gilleron M, Prandi J, Hanau D, Heck AJ, Mori L, De Libero G, Puzo G, Mourey L, de la Salle H (2011) Crystal structure of human CD1e reveals a groove suited for lipid-exchange processes. Proc Natl Acad Sci USA 108(32):13230–13235. https://doi.org/10.1073/pnas.1105627108
Zajonc DM (2016) The CD1 family: serving lipid antigens to T cells since the Mesozoic era. Immunogenetics 68(8):561–576. https://doi.org/10.1007/s00251-016-0931-0
Moody DB, Zajonc DM, Wilson IA (2005) Anatomy of CD1-lipid antigen complexes. Nat Rev Immunol 5(5):387–399. https://doi.org/10.1038/nri1605
Smith ME, Thomas JA, Bodmer WF (1988) CD1c antigens are present in normal and neoplastic B-cells. J Pathol 156(2):169–177. https://doi.org/10.1002/path.1711560212
Pena-Cruz V, Ito S, Oukka M, Yoneda K, Dascher CC, Von Lichtenberg F, Sugita M (2001) Extraction of human Langerhans cells: a method for isolation of epidermis-resident dendritic cells. J Immunol Methods 255(1–2):83–91
Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J (1992) GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature 360(6401):258–261. https://doi.org/10.1038/360258a0
de Jong A, Cheng TY, Huang S, Gras S, Birkinshaw RW, Kasmar AG, Van Rhijn I, Pena-Cruz V, Ruan DT, Altman JD, Rossjohn J, Moody DB (2014) CD1a-autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol 15(2):177–185. https://doi.org/10.1038/ni.2790
de Jong A, Pena-Cruz V, Cheng TY, Clark RA, Van Rhijn I, Moody DB (2010) CD1a-autoreactive T cells are a normal component of the human alphabeta T cell repertoire. Nat Immunol 11(12):1102–1109. https://doi.org/10.1038/ni.1956
Ly D, Moody DB (2014) The CD1 size problem: lipid antigens, ligands, and scaffolds. Cell Mol Life Sci CMLS 71(16):3069–3079. https://doi.org/10.1007/s00018-014-1603-6
Skold M, Behar SM (2003) Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun 71(10):5447–5455
Godfrey DI, McCluskey J, Rossjohn J (2005) CD1d antigen presentation: treats for NKT cells. Nat Immunol 6(8):754–756. https://doi.org/10.1038/ni0805-754
Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L (2004) NKT cells: what’s in a name? Nat Rev Immunol 4(3):231–237. https://doi.org/10.1038/nri1309
Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M (1997) CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278(5343):1626–1629
Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI (2012) Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol 12(12):845–857. https://doi.org/10.1038/nri3328
Patel O, Pellicci DG, Gras S, Sandoval-Romero ML, Uldrich AP, Mallevaey T, Clarke AJ, Le Nours J, Theodossis A, Cardell SL, Gapin L, Godfrey DI, Rossjohn J (2012) Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol 13(9):857–863. https://doi.org/10.1038/ni.2372
Girardi E, Maricic I, Wang J, Mac TT, Iyer P, Kumar V, Zajonc DM (2012) Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat Immunol 13(9):851–856. https://doi.org/10.1038/ni.2371
Brigl M, Brenner MB (2004) CD1: antigen presentation and T cell function. Annu Rev Immunol 22:817–890. https://doi.org/10.1146/annurev.immunol.22.012703.104608
Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, Kyparissoudis K, Kjer-Nielsen L, Vivian JP, Cao B, Brooks AG, Williams SJ, Illarionov P, Besra GS, Turner SJ, Porcelli SA, McCluskey J, Smyth MJ, Rossjohn J, Godfrey DI (2011) A semi-invariant Valpha10 + T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol 12(7):616–623. https://doi.org/10.1038/ni.2051
Pellicci DG, Uldrich AP, Le Nours J, Ross F, Chabrol E, Eckle SB, de Boer R, Lim RT, McPherson K, Besra G, Howell AR, Moretta L, McCluskey J, Heemskerk MH, Gras S, Rossjohn J, Godfrey DI (2014) The molecular bases of delta/alphabeta T cell-mediated antigen recognition. J Exp Med 211(13):2599–2615. https://doi.org/10.1084/jem.20141764
Zajonc DM, Girardi E (2014) A gammadelta T-cell glimpse of glycolipids. Immunol Cell Biol 92(2):99–100. https://doi.org/10.1038/icb.2013.83
Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, Godfrey DI (2013) CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 14(11):1137–1145. https://doi.org/10.1038/ni.2713
Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, Savage PB, Jabri B, Bendelac A, Adams EJ (2013) Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity 39(6):1032–1042. https://doi.org/10.1016/j.immuni.2013.11.001
Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V (2005) The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol 6(8):819–826. https://doi.org/10.1038/ni1225
Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson IA (1997) Crystal structure of mouse CD1: an MHC-like fold with a large hydrophobic binding groove. Science 277(5324):339–345
Kinjo Y, Ueno K (2011) iNKT cells in microbial immunity: recognition of microbial glycolipids. Microbiol Immunol 55(7):472–482. https://doi.org/10.1111/j.1348-0421.2011.00338.x
Zajonc DM, Girardi E (2015) Recognition of microbial glycolipids by natural killer T cells. Front Immunol 6:400. https://doi.org/10.3389/fimmu.2015.00400
Van Kaer L, Parekh VV, Wu L (2015) The response of CD1d-restricted invariant NKT cells to microbial pathogens and their products. Front Immunol 6:226. https://doi.org/10.3389/fimmu.2015.00226
Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, Hurwitz R, Kursar M, Bonneville M, Kaufmann SH, Schaible UE (2004) Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci USA 101(29):10685–10690. https://doi.org/10.1073/pnas.0403787101
Venkataswamy MM, Porcelli SA (2010) Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol 22(2):68–78. https://doi.org/10.1016/j.smim.2009.10.003
Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, Khurana A, Hoebe K, Behar SM, Beutler B, Wilson IA, Tsuji M, Sellati TJ, Wong CH, Kronenberg M (2006) Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 7(9):978–986. https://doi.org/10.1038/ni1380
An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL (2014) Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156(1–2):123–133. https://doi.org/10.1016/j.cell.2013.11.042
Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, Sonnenburg JL, Comstock LE, Bluestone JA, Fischbach MA (2013) Production of alpha-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol 11(7):e1001610. https://doi.org/10.1371/journal.pbio.1001610
Mattner J, Debord KL, Ismail N, Goff RD, Cantu C 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434(7032):525–529. https://doi.org/10.1038/nature03408
Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434(7032):520–525. https://doi.org/10.1038/nature03407
Kawahara K, Moll H, Knirel YA, Seydel U, Zahringer U (2000) Structural analysis of two glycosphingolipids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. Eur J Biochem/FEBS 267(6):1837–1846
Florence WC, Bhat RK, Joyce S (2008) CD1d-restricted glycolipid antigens: presentation principles, recognition logic and functional consequences. Expert Rev Mol Med 10:e20. https://doi.org/10.1017/S1462399408000732
Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J (2007) CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448(7149):44–49. https://doi.org/10.1038/nature05907
Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J (2009) Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity 31(1):47–59. https://doi.org/10.1016/j.immuni.2009.04.018
Wu D, Zajonc DM, Fujio M, Sullivan BA, Kinjo Y, Kronenberg M, Wilson IA, Wong CH (2006) Design of natural killer T cell activators: structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci USA 103(11):3972–3977. https://doi.org/10.1073/pnas.0600285103
Olson CM Jr, Bates TC, Izadi H, Radolf JD, Huber SA, Boyson JE, Anguita J (2009) Local production of IFN-gamma by invariant NKT cells modulates acute Lyme carditis. J Immunol 182(6):3728–3734. https://doi.org/10.4049/jimmunol.0804111
Wang J, Li Y, Kinjo Y, Mac TT, Gibson D, Painter GF, Kronenberg M, Zajonc DM (2010) Lipid binding orientation within CD1d affects recognition of Borrelia burgdorferi antigens by NKT cells. Proc Natl Acad Sci USA 107(4):1535–1540. https://doi.org/10.1073/pnas.0909479107
Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, Li Y, Imamura M, Kaneko Y, Okawara A, Miyazaki Y, Gomez-Velasco A, Rogers P, Dahesh S, Uchiyama S, Khurana A, Kawahara K, Yesilkaya H, Andrew PW, Wong CH, Kawakami K, Nizet V, Besra GS, Tsuji M, Zajonc DM, Kronenberg M (2011) Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol 12(10):966–974. https://doi.org/10.1038/ni.2096
Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, Godfrey DI, Marrack P, Rossjohn J, Gapin L (2011) A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity 34(3):315–326. https://doi.org/10.1016/j.immuni.2011.01.013
Zajonc DM, Ainge GD, Painter GF, Severn WB, Wilson IA (2006) Structural characterization of mycobacterial phosphatidylinositol mannoside binding to mouse CD1d. J Immunol 177(7):4577–4583
Giabbai B, Sidobre S, Crispin MD, Sanchez-Ruiz Y, Bachi A, Kronenberg M, Wilson IA, Degano M (2005) Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: a molecular basis for NKT cell activation. J Immunol 175(2):977–984
Lotter H, Gonzalez-Roldan N, Lindner B, Winau F, Isibasi A, Moreno-Lafont M, Ulmer AJ, Holst O, Tannich E, Jacobs T (2009) Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoeba histolytica are critically important to control amebic liver abscess. PLoS Pathog 5(5):e1000434. https://doi.org/10.1371/journal.ppat.1000434
Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K (1995) Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol 177(18):5327–5333
Ito Y, Vela JL, Matsumura F, Hoshino H, Tyznik A, Lee H, Girardi E, Zajonc DM, Liddington R, Kobayashi M, Bao X, Bugaytsova J, Boren T, Jin R, Zong Y, Seeberger PH, Nakayama J, Kronenberg M, Fukuda M (2013) Helicobacter pylori cholesteryl alpha-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PLoS One 8(12):e78191. https://doi.org/10.1371/journal.pone.0078191
Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, Kitano N, Singh A, Bhatt A, Besra GS, van den Elzen P, Appelmelk B, Franck RW, Chen G, DeKruyff RH, Shimamura M, Illarionov P, Umetsu DT (2011) Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Investig 121(1):57–69. https://doi.org/10.1172/JCI44845
Tatituri RV, Watts GF, Bhowruth V, Barton N, Rothchild A, Hsu FF, Almeida CF, Cox LR, Eggeling L, Cardell S, Rossjohn J, Godfrey DI, Behar SM, Besra GS, Brenner MB, Brigl M (2013) Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci USA 110(5):1827–1832. https://doi.org/10.1073/pnas.1220601110
Wolf BJ, Tatituri RV, Almeida CF, Le Nours J, Bhowruth V, Johnson D, Uldrich AP, Hsu FF, Brigl M, Besra GS, Rossjohn J, Godfrey DI, Brenner MB (2015) Identification of a potent microbial lipid antigen for diverse NKT cells. J Immunol 195(6):2540–2551. https://doi.org/10.4049/jimmunol.1501019
Li Y, Girardi E, Wang J, Yu ED, Painter GF, Kronenberg M, Zajonc DM (2010) The Valpha14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med 207(11):2383–2393. https://doi.org/10.1084/jem.20101335
Girardi E, Yu ED, Li Y, Tarumoto N, Pei B, Wang J, Illarionov P, Kinjo Y, Kronenberg M, Zajonc DM (2011) Unique interplay between sugar and lipid in determining the antigenic potency of bacterial antigens for NKT cells. PLoS Biol 9(11):e1001189. https://doi.org/10.1371/journal.pbio.1001189
Pellicci DG, Clarke AJ, Patel O, Mallevaey T, Beddoe T, Le Nours J, Uldrich AP, McCluskey J, Besra GS, Porcelli SA, Gapin L, Godfrey DI, Rossjohn J (2011) Recognition of beta-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat Immunol 12(9):827–833. https://doi.org/10.1038/ni.2076
Girardi E, Zajonc DM (2012) Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol Rev 250(1):167–179. https://doi.org/10.1111/j.1600-065X.2012.01166.x
De Silva AD, Park JJ, Matsuki N, Stanic AK, Brutkiewicz RR, Medof ME, Joyce S (2002) Lipid protein interactions: the assembly of CD1d1 with cellular phospholipids occurs in the endoplasmic reticulum. J Immunol 168(2):723–733
Garcia-Alles LF, Versluis K, Maveyraud L, Vallina AT, Sansano S, Bello NF, Gober HJ, Guillet V, de la Salle H, Puzo G, Mori L, Heck AJ, De Libero G, Mourey L (2006) Endogenous phosphatidylcholine and a long spacer ligand stabilize the lipid-binding groove of CD1b. EMBO J 25(15):3684–3692. https://doi.org/10.1038/sj.emboj.7601244
Huang S, Cheng TY, Young DC, Layre E, Madigan CA, Shires J, Cerundolo V, Altman JD, Moody DB (2011) Discovery of deoxyceramides and diacylglycerols as CD1b scaffold lipids among diverse groove-blocking lipids of the human CD1 system. Proc Natl Acad Sci USA 108(48):19335–19340. https://doi.org/10.1073/pnas.1112969108
Shamshiev A, Donda A, Prigozy TI, Mori L, Chigorno V, Benedict CA, Kappos L, Sonnino S, Kronenberg M, De Libero G (2000) The alphabeta T cell response to self-glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity 13(2):255–264
Moody DB, Briken V, Cheng TY, Roura-Mir C, Guy MR, Geho DH, Tykocinski ML, Besra GS, Porcelli SA (2002) Lipid length controls antigen entry into endosomal and nonendosomal pathways for CD1b presentation. Nat Immunol 3(5):435–442. https://doi.org/10.1038/ni780
Manolova V, Kistowska M, Paoletti S, Baltariu GM, Bausinger H, Hanau D, Mori L, De Libero G (2006) Functional CD1a is stabilized by exogenous lipids. Eur J Immunol 36(5):1083–1092. https://doi.org/10.1002/eji.200535544
Briken V, Jackman RM, Watts GF, Rogers RA, Porcelli SA (2000) Human CD1b and CD1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J Exp Med 192(2):281–288
Sugita M, van Der WN, Rogers RA, Peters PJ, Brenner MB (2000) CD1c molecules broadly survey the endocytic system. Proc Natl Acad Sci USA 97(15):8445–8450
Briken V, Jackman RM, Dasgupta S, Hoening S, Porcelli SA (2002) Intracellular trafficking pathway of newly synthesized CD1b molecules. EMBO J 21(4):825–834. https://doi.org/10.1093/emboj/21.4.825
Moody DB, Porcelli SA (2003) Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol 3(1):11–22
Cheng TY, Relloso M, Van Rhijn I, Young DC, Besra GS, Briken V, Zajonc DM, Wilson IA, Porcelli S, Moody DB (2006) Role of lipid trimming and CD1 groove size in cellular antigen presentation. EMBO J 25(13):2989–2999. https://doi.org/10.1038/sj.emboj.7601185
Ly D, Kasmar AG, Cheng TY, de Jong A, Huang S, Roy S, Bhatt A, van Summeren RP, Altman JD, Jacobs WR Jr, Adams EJ, Minnaard AJ, Porcelli SA, Moody DB (2013) CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med 210(4):729–741. https://doi.org/10.1084/jem.20120624
Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, Porcelli SA (2000) CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404(6780):884–888
de Jong A, Arce EC, Cheng TY, van Summeren RP, Feringa BL, Dudkin V, Crich D, Matsunaga I, Minnaard AJ, Moody DB (2007) CD1c presentation of synthetic glycolipid antigens with foreign alkyl branching motifs. Chem Biol 14(11):1232–1242. https://doi.org/10.1016/j.chembiol.2007.09.010
de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Maitre B, Mourey L, Salamero J, Cazenave JP, Hanau D, Mori L, Puzo G, De Libero G (2005) Assistance of microbial glycolipid antigen processing by CD1e. Science 310(5752):1321–1324. https://doi.org/10.1126/science.1115301
Gilleron M, Lepore M, Layre E, Cala-De Paepe D, Mebarek N, Shayman JA, Canaan S, Mori L, Carriere F, Puzo G, De Libero G (2016) Lysosomal lipases PLRP2 and LPLA2 process mycobacterial multi-acylated lipids and generate T cell stimulatory antigens. Cell Chem Biol 23(9):1147–1156. https://doi.org/10.1016/j.chembiol.2016.07.021
Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M (2001) Glycolipid antigen processing for presentation by CD1d molecules. Science 291(5504):664–667. https://doi.org/10.1126/science.291.5504.664
Relloso M, Cheng TY, Im JS, Parisini E, Roura-Mir C, DeBono C, Zajonc DM, Murga LF, Ondrechen MJ, Wilson IA, Porcelli SA, Moody DB (2008) pH-dependent interdomain tethers of CD1b regulate its antigen capture. Immunity 28(6):774–786. https://doi.org/10.1016/j.immuni.2008.04.017
Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, Glickman J, Kuo T, Little A, Morrison J, Corazza N, Kim JY, Colgan SP, Young SG, Exley M, Blumberg RS (2004) CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med 10(5):535–539. https://doi.org/10.1038/nm1043
Zhou D, Cantu C 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, Savage P, Bendelac A, Teyton L (2004) Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science 303(5657):523–527. https://doi.org/10.1126/science.1092009
Yuan W, Qi X, Tsang P, Kang SJ, Illarionov PA, Besra GS, Gumperz J, Cresswell P (2007) Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci USA 104(13):5551–5556. https://doi.org/10.1073/pnas.0700617104
Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, Porcelli SA, Brinkmann V, Sugita M, Sandhoff K, Kaufmann SH, Schaible UE (2004) Saposin C is required for lipid presentation by human CD1b. Nat Immunol 5(2):169–174. https://doi.org/10.1038/ni1035
Cala-De Paepe D, Layre E, Giacometti G, Garcia-Alles LF, Mori L, Hanau D, de Libero G, de la Salle H, Puzo G, Gilleron M (2012) Deciphering the role of CD1e protein in mycobacterial phosphatidyl-myo-inositol mannosides (PIM) processing for presentation by CD1b to T lymphocytes. J Biol Chem 287(37):31494–31502. https://doi.org/10.1074/jbc.M112.386300
Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, Porcelli SA, Brenner MB (1999) Molecular recognition of lipid antigens by T cell receptors. J Exp Med 189(1):195–205
Van Rhijn I, Young DC, Im JS, Levery SB, Illarionov PA, Besra GS, Porcelli SA, Gumperz J, Cheng TY, Moody DB (2004) CD1d-restricted T cell activation by nonlipidic small molecules. Proc Natl Acad Sci USA 101(37):13578–13583. https://doi.org/10.1073/pnas.0402838101
Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, Rossjohn J, Moody DB (2013) A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol 14(7):706–713. https://doi.org/10.1038/ni.2630
Van Rhijn I, Gherardin NA, Kasmar A, de Jager W, Pellicci DG, Kostenko L, Tan LL, Bhati M, Gras S, Godfrey DI, Rossjohn J, Moody DB (2014) TCR bias and affinity define two compartments of the CD1b-glycolipid-specific T cell repertoire. J Immunol 193(10):5338–5344. https://doi.org/10.4049/jimmunol.1400158
Turner SJ, Doherty PC, McCluskey J, Rossjohn J (2006) Structural determinants of T-cell receptor bias in immunity. Nat Rev Immunol 6(12):883–894. https://doi.org/10.1038/nri1977
Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB (2000) Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med 191(6):937–948
Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, Spinozzi F (2007) CD1-restricted recognition of exogenous and self-lipid antigens by duodenal gammadelta + T lymphocytes. J Immunol 178(6):3620–3626
Roy S, Ly D, Castro CD, Li NS, Hawk AJ, Altman JD, Meredith SC, Piccirilli JA, Moody DB, Adams EJ (2016) Molecular analysis of lipid-reactive vdelta1 gammadelta T cells identified by CD1c tetramers. J Immunol 196(4):1933–1942. https://doi.org/10.4049/jimmunol.1502202
Kasmar A, Van Rhijn I, Magalhaes KG, Young D, Cheng TY, Turner M, Schiefner A, Kalathur RC, Wilson A, Bhati M, Gras S, Rossjohn J, Shires J, Jakobsen S, Altman JD, Moody DB (2013) CD1a tetramers and dextramers identify human lipopeptide-specific T cells ex vivo. J Immunol 191:4499–4503
Van Rhijn I, Iwany SK, Fodran P, Cheng TY, Gapin L, Minnaard AJ, Moody DB (2017) CD1b-mycolic acid tetramers demonstrate T-cell fine specificity for mycobacterial lipid tails. Eur J Immunol. https://doi.org/10.1002/eji.201747062
Seshadri C, Lin L, Scriba TJ, Peterson G, Freidrich D, Frahm N, DeRosa SC, Moody DB, Prandi J, Gilleron M, Mahomed H, Jiang W, Finak G, Hanekom WA, Gottardo R, McElrath MJ, Hawn TR (2015) T cell responses against mycobacterial lipids and proteins are poorly correlated in South African adolescents. J Immunol 195(10):4595–4603. https://doi.org/10.4049/jimmunol.1501285
Felio K, Nguyen H, Dascher CC, Choi HJ, Li S, Zimmer MI, Colmone A, Moody DB, Brenner MB, Wang CR (2009) CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med 206(11):2497–2509. https://doi.org/10.1084/jem.20090898
Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, McMurray DN, LeClair KP, Porcelli SA, Brenner MB (2003) Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int Immunol 15(8):915–925
Porcelli S, Morita CT, Brenner MB (1992) CD1b restricts the response of human CD4-8-T lymphocytes to a microbial antigen. Nature 360(6404):593–597. https://doi.org/10.1038/360593a0
Moody DB, Ulrichs T, Muhlecker W, Young DC, Gurcha SS, Grant E, Rosat JP, Brenner MB, Costello CE, Besra GS, Porcelli SA (2000) CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404(6780):884–888. https://doi.org/10.1038/35009119
Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ et al (1995) CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269(5221):227–230
Ernst WA, Maher J, Cho S, Niazi KR, Chatterjee D, Moody DB, Besra GS, Watanabe Y, Jensen PE, Porcelli SA, Kronenberg M, Modlin RL (1998) Molecular interaction of CD1b with lipoglycan antigens. Immunity 8(3):331–340
Takayama K, Wang C, Besra GS (2005) Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin Microbiol Rev 18(1):81–101. https://doi.org/10.1128/CMR.18.1.81-101.2005
Grant EP, Beckman EM, Behar SM, Degano M, Frederique D, Besra GS, Wilson IA, Porcelli SA, Furlong ST, Brenner MB (2002) Fine specificity of TCR complementarity-determining region residues and lipid antigen hydrophilic moieties in the recognition of a CD1-lipid complex. J Immunol 168(8):3933–3940
Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, Besra GS, Porcelli SA (1997) Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 278(5336):283–286
Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, Rossjohn J, Moody DB (2013) A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol 14(7):706–713. https://doi.org/10.1038/ni.2630
Van Rhijn I, Moody DB (2015) CD1 and mycobacterial lipids activate human T cells. Immunol Rev 264(1):138–153. https://doi.org/10.1111/imr.12253
Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, Gilleron M (2009) Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol 16(1):82–92. https://doi.org/10.1016/j.chembiol.2008.11.008
Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, Leon L, Brenner M, Wilson IA, Altman JD, Moody DB (2011) CD1b tetramers bind alphabeta T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med 208(9):1741–1747. https://doi.org/10.1084/jem.20110665
Gras S, Van Rhijn I, Shahine A, Cheng TY, Bhati M, Tan LL, Halim H, Tuttle KD, Gapin L, Le Nours J, Moody DB, Rossjohn J (2016) T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat Commun 7:13257. https://doi.org/10.1038/ncomms13257
Batuwangala T, Shepherd D, Gadola SD, Gibson KJ, Zaccai NR, Fersht AR, Besra GS, Cerundolo V, Jones EY (2004) The crystal structure of human CD1b with a bound bacterial glycolipid. J Immunol 172(4):2382–2388
Garcia-Alles LF, Collmann A, Versluis C, Lindner B, Guiard J, Maveyraud L, Huc E, Im JS, Sansano S, Brando T, Julien S, Prandi J, Gilleron M, Porcelli SA, de la Salle H, Heck AJ, Mori L, Puzo G, Mourey L, De Libero G (2011) Structural reorganization of the antigen-binding groove of human CD1b for presentation of mycobacterial sulfoglycolipids. Proc Natl Acad Sci USA 108(43):17755–17760. https://doi.org/10.1073/pnas.1110118108
Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Bohmer G, Prandi J, Mori L, Puzo G, De Libero G (2004) Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med 199(5):649–659. https://doi.org/10.1084/jem.20031097
Moody DB, Young DC, Cheng TY, Rosat JP, Roura-Mir C, O’Connor PB, Zajonc DM, Walz A, Miller MJ, Levery SB, Wilson IA, Costello CE, Brenner MB (2004) T cell activation by lipopeptide antigens. Science 303(5657):527–531. https://doi.org/10.1126/science.1089353
Zajonc DM, Crispin MD, Bowden TA, Young DC, Cheng TY, Hu J, Costello CE, Rudd PM, Dwek RA, Miller MJ, Brenner MB, Moody DB, Wilson IA (2005) Molecular mechanism of lipopeptide presentation by CD1a. Immunity 22(2):209–219. https://doi.org/10.1016/j.immuni.2004.12.009
Snow GA, White AJ (1969) Chemical and biological properties of mycobactins isolated from various mycobacteria. Biochem J 115(5):1031–1050
Layre E, Paepe DC, Larrouy-Maumus G, Vaubourgeix J, Mundayoor S, Lindner B, Puzo G, Gilleron M (2011) Deciphering sulfoglycolipids of Mycobacterium tuberculosis. J Lipid Res 52(6):1098–1110. https://doi.org/10.1194/jlr.M013482
Scharf L, Li NS, Hawk AJ, Garzon D, Zhang T, Fox LM, Kazen AR, Shah S, Haddadian EJ, Gumperz JE, Saghatelian A, Faraldo-Gomez JD, Meredith SC, Piccirilli JA, Adams EJ (2010) The 2.5 A structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity 33(6):853–862. https://doi.org/10.1016/j.immuni.2010.11.026
Roy S, Ly D, Li NS, Altman JD, Piccirilli JA, Moody DB, Adams EJ (2014) Molecular basis of mycobacterial lipid antigen presentation by CD1c and its recognition by alphabeta T cells. Proc Natl Acad Sci USA 111(43):E4648–E4657. https://doi.org/10.1073/pnas.1408549111
Van Rhijn I, van Berlo T, Hilmenyuk T, Cheng TY, Wolf BJ, Tatituri RV, Uldrich AP, Napolitani G, Cerundolo V, Altman JD, Willemsen P, Huang S, Rossjohn J, Besra GS, Brenner MB, Godfrey DI, Moody DB (2016) Human autoreactive T cells recognize CD1b and phospholipids. Proc Natl Acad Sci USA 113(2):380–385. https://doi.org/10.1073/pnas.1520947112
Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, Schmidt RR, Jones EY, Cerundolo V (2002) Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat Immunol 3(8):721–726. https://doi.org/10.1038/ni821
Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J (2006) CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic acids research 34(Web Server issue):W116–W118. https://doi.org/10.1093/nar/gkl282
Schrodinger, LLC (2015) The PyMOL Molecular Graphics System, Version 1.7.6.4
Acknowledgements
Our research is supported by the US National Institutes of Health (NIH) (AI 111224), Monash University (Australia), the Australian National Health and Medical Research (NHMRC), and the Australian Research Council (ARC). SG is a Monash Senior Research Fellow and J. L. N. is an ARC Future Fellow (FT160100074).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gras, S., Van Rhijn, I., Shahine, A. et al. Molecular recognition of microbial lipid-based antigens by T cells. Cell. Mol. Life Sci. 75, 1623–1639 (2018). https://doi.org/10.1007/s00018-018-2749-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2749-4