Abstract
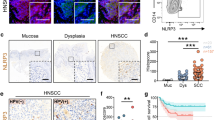
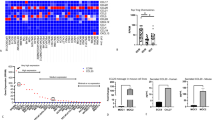
The NLRP3 inflammasome is a critical innate immune pathway responsible for producing active interleukin (IL)-1β, which is associated with tumor development and immunity. However, the mechanisms regulating the inflammatory microenvironment, tumorigenesis and tumor immunity are unclear. Herein, we show that the NLRP3 inflammasome was over-expressed in human HNSCC tissues and that the IL-1β concentration was increased in the peripheral blood of HNSCC patients. Additionally, elevated NLRP3 inflammasome levels were detected in tumor tissues of Tgfbr1/Pten 2cKO HNSCC mice, and elevated IL-1β levels were detected in the peripheral blood serum, spleen, draining lymph nodes and tumor tissues. Blocking NLRP3 inflammasome activation using MCC950 remarkably reduced IL-1β production in an HNSCC mouse model and reduced the numbers of myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and tumor-associated macrophages (TAMs). Moreover, inhibiting NLRP3 inflammasome activation increased the numbers of CD4+ and CD8+ T cells in HNSCC mice. Notably, the numbers of exhausted PD-1+ and Tim3+ T cells were significantly reduced. A human HNSCC tissue microarray showed that NLRP3 inflammasome expression was correlated with the expression of CD8 and CD4, the Treg marker Foxp3, the MDSC markers CD11b and CD33, and the TAM markers CD68 and CD163, PD-1 and Tim3. Overall, our results demonstrate that the NLRP3 inflammasome/IL-1β pathway promotes tumorigenesis in HNSCC and inactivation of this pathway delays tumor growth, accompanied by decreased immunosuppressive cell accumulation and an increased number of effector T cells. Thus, inhibition of the tumor microenvironment through the NLRP3 inflammasome/IL-1β pathway may provide a novel approach for HNSCC therapy.







Similar content being viewed by others
Abbreviations
- NLRP3:
-
Nod-like receptor protein 3
- HNSCC:
-
Head and neck squamous cell carcinoma
- Dys:
-
Dysplasia
- IL-1β:
-
Interleukin (IL)-1β
- TILs:
-
Tumor-infiltrating lymphocytes
- Tregs:
-
Regulatory T cells
- TCGA:
-
The Cancer Genome Atlas
- MDSCs:
-
Myeloid-derived suppressor cells
- TAMs:
-
Tumor-associated macrophages
- PD-1:
-
Programmed death-1
- Tim3:
-
T cell immunoglobulin mucin-3
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140(6):883–899
Coussens LM, Zitvogel L, Palucka AK (2013) Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 339(6117):286–291
Próchnicki T, Mangan MS, Latz E (2016) Recent insights into the molecular mechanisms of the NLRP3 inflammasome activation. F1000Res 5:1469
Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W (2014) Inflammasomes in cancer: a double-edged sword. Protein Cell 5(1):12–20
Menu P, Vince JE (2011) The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol 166(1):1
Li Y, Wang L, Pappan L, Galliher-beckley A, Shi J (2012) IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Mol Cancer 11(1):87
Abrahamsson A, Morad V, Saarinen NM, Dabrosin C (2012) Estradiol, tamoxifen, and flaxseed alter IL-1β and IL-1Ra levels in normal human breast tissue in vivo. J Clin Endocrinol Metab 97(11):2044–2054
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in China. CA Cancer J Clin 66(2):115–132
Hunter KD, Parkinson EK, Harrison PR (2005) Profiling early head and neck cancer. Nat Rev Cancer 5(2):127–135
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45(4–5):309
Ferris RL (2015) Immunology and immunotherapy of head and neck cancer. J Clin Oncol 33(29):3293
Ferris RL, Whiteside TL, Ferrone S (2006) Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res 12(13):3890–3895
Dasgupta S, Bhattacharyachatterjee M, O’Malley BW Jr, Chatterjee SK (2005) Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J Immunol 175(8):5541–5550
Kuss I, Hathaway B, Ferris RL, Gooding W, Whiteside TL (2004) Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck. Clin Cancer Res 10(11):3755–3762
Economopoulou P, Kotsantis I, Psyrri A (2016) Checkpoint inhibitors in head and neck cancer: rationale, clinical activity, and potential biomarkers. Curr Treat Options Oncol 17(8):40
Guo B, Fu S, Zhang J, Liu B, Li Z (2016) Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep 6:36107
Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S (2013) Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol 25(2):268–276
Kanterman J, Sadefeldman M, Baniyash M (2012) New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol 22(4):307–318
Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL (2013) Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 19(1):57–64
Yu GT, Bu LL, Zhao YY, Mao L, Deng WW, Wu TF, Zhang WF, Sun ZJ (2016) CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology 5(6):e1151594
Deng WW, Mao L, Yu GT, Bu LL, Ma SR, Liu B, Gutkind JS, Kulkarni AB, Zhang WF, Sun ZJ (2016) LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology 5(11):e1239005
Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM, Kumari S, Kelly RL, Kwan BH, Abraham W, Hu K, Mehta NK, Kauke MJ, Suh H, Cochran JR, Lauffenburger DA, Wittrup KD, Irvine DJ (2016) Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med 22(12):1402–1410
Yu GT, Bu LL, Huang CF, Zhang WF, Chen WJ, Gutkind JS, Kulkarni AB, Sun ZJ (2015) PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPα axis in HPV negative head and neck squamous cell carcinoma. Oncotarget 6(39):42067–42080
Bian Y, Hall B, Sun ZJ, Molinolo A, Chen W, Gutkind JS, Waes CV, Kulkarni AB (2012) Loss of TGF-β signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene 31(28):3322–3332
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95(25):14863–14868
Saldanha AJ (2004) Java Treeview—extensible visualization of microarray data. Bioinformatics 20(17):3246–3248
Peng CH, Liao CT, Peng SC, Chen YJ, Cheng AJ, Juang JL, Tsai CY, Chen TC, Chuang YJ, Tang CY, Hsieh WP, Yen TC (2011) A novel molecular signature identified by systems genetics approach predicts prognosis in oral squamous cell carcinoma. PLoS One 6(8):e23452
Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X (2008) Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genom 9(1):69
Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL, Gaffney PM (2004) Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res 64(1):55
Putz G, Rosner AI, Schmitz N, Buchholz F (2006) AML1 deletion in adult mice causes splenomegaly and lymphomas. Oncogene 25(6):929–939
Xie P, Stunz LL, Larison KD, Yang B, Bishop GA (2007) TRAF3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity 27(2):253–267
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6(4):295
Je-In Youn SN, Collazo Michelle, Gabrilovich Dmitry I (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 181(8):5791–5802
Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P (2016) CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res 76(19):5671
Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Müller M, Blander JM, Tacke F, Trautwein C (2010) Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 207(7):1453–1464
Mccracken MN, Cha AC, Weissman IL (2015) Molecular pathways: activating T cells after cancer cell phagocytosis from blockade of CD47 “don’t eat me” signals. Clin Cancer Res 21(16):3597–3601
Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C (2015) NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 6:262
Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V (2014) Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 1319(1):47–65
Yang X, Lu H, Yan B, Romano RA, Bian Y, Friedman J, Duggal P, Allen C, Chuang R, Ehsanian R (2011) ΔNp63 versatilely regulates a Broad NF-κB gene program and promotes squamous epithelial proliferation, migration, and inflammation. Cancer Res 71(10):3688–3700
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21(7):677
Huang CF, Chen L, Li YC, Wu L, Yu GT, Zhang WF, Sun ZJ (2017) NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res 36(1):116
Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, Dinarello CA, Fujita M (2010) Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem 285(9):6477–6488
Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, Ting JP (2010) The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med 207(5):1045–1056
Zaki MH, Vogel P, Bodymalapel M, Lamkanfi M, Kanneganti TD (2010) IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J Immunol 185(8):4912
Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KSB, Meunier C, Gros P, Vallance BA, Saleh M, Mcintire CR, Leblanc PM (2010) Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32(3):367
Fabbi M, Carbotti G, Ferrini S (2015) Context-dependent role of IL-18 in cancer biology and counter-regulation by IL-18BP. J Leukoc Biol 97(4):665
Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC (2011) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14(5):408–419
Terlizzi M, Colarusso C, Popolo A, Pinto A, Sorrentino R (2016) IL-1α and IL-1β-producing macrophages populate lung tumor lesions in mice. Oncotarget 7(36):58181–58182
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 136(10):2352–2360
Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di SJ, Apte RN, Vosshenrich CA (2010) IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol 40(12):3347–3357
Balermpas P, Rödel F, Liberz R, Oppermann J, Wagenblast J, Ghanaati S, Harter PN, Mittelbronn M, Weiss C, Rödel C (2014) Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer 111(8):1509
Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, Frank A, Scholich K, Pierre S, Syed S (2017) S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1β. J Exp Med 214(9):2695–2713
Zarif JC, Taichman RS, Pienta KJ (2014) TAM macrophages promote growth and metastasis within the cancer ecosystem. Oncoimmunology 3(7):e941734
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Acknowledgements
This work was supported by National Natural Science Foundation of China 81402241, 81672667, 81672668, 81472528, 81472529.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
Animal studies were approved and supervised by the Animal Care and Use Committee of Wuhan University. All human studies obtained informed consent from patients at the beginning of the trial and were approved by the Medical Ethics Committee of the Hospital of Stomatology, Wuhan University. The ethical approval number is 2014C66.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
18_2017_2720_MOESM1_ESM.tif
Supplementary Fig. 1. a IL-1B mRNA expression levels from several datasets for Head–Neck cancer versus normal mucosa counterpart. b IL-1B mRNA expression was closely related to NLRP3 in TCGA dataset (p < 0.001, r = 0.3558) (TIFF 1065 kb)
18_2017_2720_MOESM2_ESM.tif
Supplementary Fig. 2. The expression of ASC, caspase-1, and IL-18 were correlated with CD8, PD-1, Tim3 and Treg, MDSC, TAM markers in human HNSCC (TIFF 11065 kb)
Rights and permissions
About this article
Cite this article
Chen, L., Huang, CF., Li, YC. et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell. Mol. Life Sci. 75, 2045–2058 (2018). https://doi.org/10.1007/s00018-017-2720-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2720-9