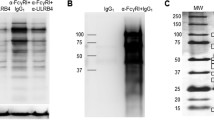
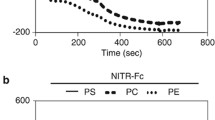
Abstract
Phagocytosis mediated by the complement receptor CR3 (also known as integrin αMß2 or Mac-1) is regulated by the recruitment of talin to the cytoplasmic tail of the ß2 integrin subunit. Talin recruitment to this integrin is dependent on Rap1 activation. However, the mechanism by which Rap1 regulates this event and CR3-dependent phagocytosis remains largely unknown. In the present work, we examined the role of the Rap1 effector RIAM, a talin-binding protein, in the regulation of complement-mediated phagocytosis. Using the human myeloid cell lines HL-60 and THP-1, we determined that knockdown of RIAM impaired αMß2 integrin affinity changes induced by stimuli fMLP and LPS. Phagocytosis of complement-opsonized RBC particles, but not of IgG-opsonized RBC particles, was impaired in RIAM knockdown cells. Rap1 activation via EPAC induced by 8-pCPT-2′-O-Me-cAMP resulted in an increase of complement-mediated phagocytosis that was abrogated by knockdown of RIAM in HL-60 and THP-1 cell lines and in macrophages derived from primary monocytes. Furthermore, recruitment of talin to ß2 integrin during complement-mediated phagocytosis was reduced in RIAM knockdown cells. These results indicate that RIAM is a critical component of the phagocytosis machinery downstream of Rap1 and mediates its function by recruiting talin to the phagocytic complement receptors.








Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- 8CPT:
-
8-pCPT-2-O-Me-cAMP
- FCS:
-
Fetal calf serum
- HRP:
-
Horseradish peroxidase
- PBMC:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate buffered saline
- PMA:
-
Phorbol myristate acetate
- RA:
-
Retinoic acid
- FNG:
-
Fibrinogen
- mAb:
-
Monoclonal antibody
- TBS:
-
Tris buffered saline
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Underhill DM, Ozinsky A (2002) Phagocytosis of microbes: complexity in action. Annu Rev Immunol 20:825–852
Caron E, Self A, Hall A (2000) The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol 10:974–978
Abram C, Lowell C (2009) The ins and outs of leukocyte integrin signaling. Annu Rev Immunol 27:339–362
May RC, Caron E, Hall A, Machesky LM (2000) Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat Cell Biol 2:246–248
Greenberg S, Burridge K, Silverstein SC (1990) Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med 172:1853–1856
Dupuy AG, Caron E (2008) Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci 121:1773–1783
Liu L, Schwartz B, Tupper J, Lin N, Winn R, Harlan J (2002) The GTPase Rap1 regulates phorbol 12-myristate 13-acetate-stimulated but not ligand-induced beta 1 integrin-dependent leukocyte adhesion. J Biol Chem 277:40893–40900
Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y et al (2000) The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol 148:1151–1158
Enserink J, Price L, Methi T, Mahic M, Sonnenberg A, Bos J et al (2004) The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to. J Biol Chem 279:44889–44896
Han J, Lim C, Watanabe N, Soriani A, Ratnikov B, Calderwood D et al (2006) Reconstructing and deconstructing agonist-induced activation of integrin. Curr Biol 16:1796–1806
Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T (2000) Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol 20:1956–1969
Lafuente E, van Puijenbroek A, Krause M, Carman C, Freeman G, Berezovskaya A et al (2004) RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates. Dev Cell 7:585–595
Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different. Science 282:1717–1721
Liu S, Calderwood DA, Ginsberg MH (2000) Integrin cytoplasmic domain-binding proteins. J Cell Sci 113(Pt 20):3563–3571
Tadokoro S, Shattil S, Eto K, Tai V, Liddington R, de Pereda J et al (2003) Talin binding to integrin beta tails: a final common step in integrin activation. Science 302:103–106
Kim M, Carman CV, Springer TA (2003) Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301:1720–1725
Lim J, Wiedemann A, Tzircotis G, Monkley S, Critchley D, Caron E (2007) An essential role for talin during alpha(M)beta(2)-mediated phagocytosis. Mol Biol Cell 18:976–985
Lim J, Dupuy A, Critchley D, Caron E (2010) Rap1 controls activation of the alpha(M)beta(2) integrin in a talin-dependent manner. J Cell Biochem 111:999–1009
Ménasché G, Kliche S, Chen E, Stradal T, Schraven B, Koretzky G (2007) RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol 27:4070–4081
Patsoukis N, Lafuente EM, Meraner P, Kim JS, Dombkowski D, Li L, Boussiotis VA (2009) RIAM regulates the cytoskeletal distribution and activation of PLC-gamma1 in T cells. Sci Signal 2(99):ra79
Watanabe N, Bodin L, Pandey M, Krause M, Coughlin S, Boussiotis V et al (2008) Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin alphaIIbbeta3. J Cell Biol 181:1211–1222
Lee H, Lim C, Puzon-McLaughlin W, Shattil S, Ginsberg M (2009) RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem 284:5119–5127
Hernandez-Varas P, Colo G, Bartolome R, Paterson A, Medraño-Fernandez I, Arellano-Sanchez N et al (2011) Rap1-GTP-interacting adaptor molecule (RIAM) protein controls invasion and growth of melanoma cells. J Biol Chem 286:18492–18504
Fleck R, Athwal H, Bygraves J, Hockley D, Feavers I, Stacey G (2003) Optimization of NB-4 and HL-60 differentiation for use in opsonophagocytosis assays. In Vitro Cell Dev Biol Anim 39:235–242
Olazabal I, Caron E, May R, Schilling K, Knecht D, Machesky L (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR phagocytosis. Curr Biol 12:1413–1418
Pricop L, Salmon J, Edberg J, Beavis A (1997) Flow cytometric quantitation of attachment and phagocytosis in phenotypically-defined subpopulations of cells using PKH26-labeled Fc gamma R-specific probes. J Immunol Methods 205:55–65
Inagaki T, Suzuki S, Miyamoto T, Takeda T, Yamashita K, Komatsu A et al (2003) The retinoic acid-responsive proline-rich protein is identified in promyeloleukemic HL-60 cells. J Biol Chem 278:51685–51692
Romero-Steiner S, Libutti D, Pais L, Dykes J, Anderson P, Whitin J et al (1997) Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 4:415–422
Rosmarin AG, Weil SC, Rosner GL, Griffin JD, Arnaout MA, Tenen DG (1989) Differential expression of CD11b/CD18 (Mo1) and myeloperoxidase genes during myeloid differentiation. Blood 73:131–136
Aragones J, Lopez-Rodriguez C, Corbi A, del Arco PG, Lopez-Cabrera M, de Landazuri MO et al (1996) Dithiocarbamates trigger differentiation and induction of CD11c gene through AP-1 in the myeloid lineage. J Biol Chem 271:10924–10931
Bertoni A, Tadokoro S, Eto K, Pampori N, Parise L, White G et al (2002) Relationships between Rap1b, affinity modulation of integrin alpha IIbbeta 3, and the actin cytoskeleton. J Biol Chem 277:25715–25721
Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell D (2002) Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol 3:251–258
Endemann G, Feng Y, Bryant CM, Hamilton GS, Perumattam J, Mewshaw RE et al (1996) Novel anti-inflammatory compounds prevent CD11b/CD18, alpha M beta 2 (Mac-1)-dependent neutrophil adhesion without blocking activation-induced changes in Mac-1. J Pharmacol Exp Ther 276:5–12
Enserink J, Christensen A, de Rooij J, van Triest M, Schwede F, Genieser H et al (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906
Misra U, Kaczowka S, Pizzo S (2008) The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-CPT-2-O-Me-cAMP-stimulated macrophages: gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-CPT-2-O-Me-cAMP activation of Akt activity in macrophages. Cell Signal 20:1459–1470
Dash-Koney M, Deevi R, McFarlane C, Dib K (2011) Exchange protein directly activated by cAMP 1 (Epac1) is expressed in human neutrophils and mediates cAMP-dependent activation of the monomeric GTPase Rap1. J Leukoc Biol 90:741–749
Lorenowicz M, van Gils J, de Boer M, Hordijk P, Fernandez-Borja M (2006) Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol 80:1542–1552
Lim J, Hotchin N, Caron E (2011) Ser756 of beta2 integrin controls Rap1 activity during inside-out activation of αMβ2. Biochem J 437:461–467
Grinstein S, Mack E, Mills GB (1986) Osmotic activation of the Na+/H+ antiport in protein kinase C-depleted lymphocytes. Biochem Biophys Res Commun 134:8–13
Rodriguez-Pena A, Rozengurt E (1984) Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun 120:1053–1059
Acknowledgments
We are thankful to Joaquin Teixidó and Georgina Coló (CIB; CSIC, Spain) for providing information about RIAM siRNA oligonucleotides, and to José Luis Rodríguez and Jesus Torres (CIB; CSIC, Spain) for their help with siRNA delivery into MDM cells. This work was supported by grants SAF2007-60578 and SAF2012-34561 from MICINN and grants CCG10-UCM/BIO-4795 and CCG08-UCM/SAL-4259 from Comunidad Autonoma de Madrid (to E.M.L.), grants BFU2007-66443/BMC and BFU2010-19144/BMC and SAF2012-34561 from MICINN (to CC), grant SAF2009-08103 from MICINN (to P.A.R.), NIH grants HL107997-01 and R56AI43552 and the Leukemia and Lymphoma Society Translational Research Program TRP 6222-11 (to V.A.B.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Medraño-Fernandez, I., Reyes, R., Olazabal, I. et al. RIAM (Rap1-interacting adaptor molecule) regulates complement-dependent phagocytosis. Cell. Mol. Life Sci. 70, 2395–2410 (2013). https://doi.org/10.1007/s00018-013-1268-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1268-6