Abstract
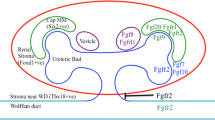
The mammalian kidney develops from the ureteric bud and the metanephric mesenchyme. In mice, the ureteric bud invades the metanephric mesenchyme at day E10.5 and begins to branch. The tips of the ureteric bud induce the metanephric mesenchyme to condense and form the cap mesenchyme. Some cells of this cap mesenchyme undergo a mesenchymal-to-epithelial transition and differentiate into renal vesicles, which further develop into nephrons. The developing kidney expresses Fibroblast growth factor (Fgf)1, 7, 8, 9, 10, 12 and 20 and Fgf receptors Fgfr1 and Fgfr2. Fgf7 and Fgf10, mainly secreted by the metanephric mesenchyme, bind to Fgfr2b of the ureteric bud and induce branching. Fgfr1 and Fgfr2c are required for formation of the metanephric mesenchyme, however the two receptors can substitute for one another. Fgf8, secreted by renal vesicles, binds to Fgfr1 and supports survival of cells in the nascent nephrons. Fgf9 and Fgf20, expressed in the metanephric mesenchyme, are necessary to maintain survival of progenitor cells in the cortical region of the kidney. FgfrL1 is a novel member of the Fgfr family that lacks the intracellular tyrosine kinase domain. It is expressed in the ureteric bud and all nephrogenic structures. Targeted deletion of FgfrL1 leads to severe kidney dysgenesis due to the lack of renal vesicles. FgfrL1 is known to interact mainly with Fgf8. It is therefore conceivable that FgfrL1 restricts signaling of Fgf8 to the precise location of the nascent nephrons. It might also promote tight adhesion of cells in the condensed metanephric mesenchyme as required for the mesenchymal-to-epithelial transition.





Similar content being viewed by others
References
Costantini F, Kopan R (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18:698–712
Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH (2009) Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332:273–286
Hendry C, Rumballe B, Moritz K, Little MH (2011) Defining and redefining the nephron progenitor population. Pediatr Nephrol 26:1395–1406
Park JS, Valerius MT, McMahon AP (2007) Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134:2533–2539
Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR (2005) FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132:3847–3857
Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M (2005) Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132:3859–3871
Stark K, Vainio S, Vassileva G, McMahon AP (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372:679–683
Wang P, Pereira FA, Beasley D, Zheng H (2003) Presenilins are required for the formation of comma- and s-shaped bodies during nephrogenesis. Development 130:5019–5029
Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R (2007) Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134:801–811
Itoh N (2007) The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull 30:1819–1825
Beenken A, Mohammadi M (2009) The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov 8:235–253
Dailey L, Ambrosetti D, Mansukhani A, Basilico C (2005) Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev 16:233–247
Lew ED, Furdui CM, Anderson KS, Schlessinger J (2009) The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci Signal 2:ra6
Wiedemann M, Trueb B (2000) Characterization of a novel protein (FGFRL1) from human cartilage related to FGF receptors. Genomics 69:275–279
Trueb B (2011) Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell Mol Life Sci 68:951–964
Bertrand S, Somorjai I, Garcia-Fernandez J, Lamonerie T, Escriva H (2009) FGFRL1 is a neglected putative actor of the FGF signalling pathway present in all major metazoan phyla. BMC Evol Biol 9:226
Zhuang L, Karotki AV, Bruecker P, Trueb B (2009) Comparison of the receptor FGFRL1 from sea urchins and humans illustrates evolution of a zinc binding motif in the intracellular domain. BMC Biochem 10:33
Zhuang L, Villiger P, Trueb B (2011) Interaction of the receptor FGFRL1 with the negative regulator Spred1. Cell Signal 23:1496–1504
Rieckmann T, Zhuang L, Flück CE, Trueb B (2009) Characterization of the first FGFRL1 mutation identified in a craniosynostosis patient. Biochim Biophys Acta 1792:112–121
Steinberg F, Zhuang L, Beyeler M, Kalin RE, Mullis PE, Brandli AW, Trueb B (2010) The FGFRL1 receptor is shed from cell membranes, binds FGFs and antagonizes FGF signaling in Xenopus embryos. J Biol Chem 285:2193–2202
Trueb B, Zhuang L, Taeschler S, Wiedemann M (2003) Characterization of FGFRL1, a novel FGF receptor preferentially expressed in skeletal tissues. J Biol Chem 278:33857–33865
Rieckmann T, Kotevic I, Trueb B (2008) The cell surface receptor FGFRL1 forms constitutive dimers that promote cell adhesion. Exp Cell Res 314:1071–1081
Steinberg F, Gerber S, Rieckmann T, Trueb B (2010) Rapid fusion and syncytium formation of heterologous cells upon expression of the FGFRL1 receptor. J Biol Chem 285:37704–37715
Sleeman M, Fraser J, McDonald M, Yuan S, White D, Grandison P, Kumble K, Watson JD, Murison JG (2001) Identification of a new fibroblast growth factor receptor, FGFR5. Gene 271:171–182
Chen EH, Grote E, Mohler W, Vignery A (2007) Cell-cell fusion. FEBS Lett 581:2181–2193
Oren-Suissa M, Podbilewicz B (2010) Evolution of programmed cell fusion: common mechanisms and distinct functions. Dev Dyn 239:1515–1528
Cancilla B, Ford-Perriss MD, Bertram JF (1999) Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int 56:2025–2039
Brown AC, Adams D, de Caestecker M, Yang X, Friesel R, Oxburgh L (2011) FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development. Development 138:5099–5112
Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D (1997) Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol 273:F757–F767
Perantoni AO, Dove LF, Karavanova I (1995) Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci USA 92:4696–4700
Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA (1999) Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99:377–386
Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO (2001) TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128:1045–1057
Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK (2001) Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev 109:123–135
Nguyen HQ, Danilenko DM, Bucay N, DeRose ML, Van GY, Thomason A, Simonet WS (1996) Expression of keratinocyte growth factor in embryonic liver of transgenic mice causes changes in epithelial growth and differentiation resulting in polycystic kidneys and other organ malformations. Oncogene 12:2109–2119
Miller DL, Ortega S, Bashayan O, Basch R, Basilico C (2000) Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol 20:2260–2268
Guo L, Degenstein L, Fuchs E (1996) Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10:165–175
Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D (1999) FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126:547–554
Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS (1998) Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev 12:3156–3161
Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S (1999) Fgf10 is essential for limb and lung formation. Nat Genet 21:138–141
Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N (2000) FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277:643–649
Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D’Agati V, Licht JD, Martin GR, Costantini F (2010) Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet 6:e1000809
Sun X, Meyers EN, Lewandoski M, Martin GR (1999) Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev 13:1834–1846
Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschke P, Salomon R, Antignac C et al (2012) FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22:1191–1207
Colvin JS, White AC, Pratt SJ, Ornitz DM (2001) Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development 128:2095–2106
Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM (2001) Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell 104:875–889
Huh SH, Jones J, Warchol ME, Ornitz DM (2012) Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol 10:e1001231
Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P (1994) Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8:3045–3057
Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J (1994) Fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev 8:3032–3044
Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C (1998) Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125:753–765
Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P (1998) Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA 95:5082–5087
Revest JM, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C (2001) Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol 231:47–62
Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G (1998) Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J 17:1642–1655
Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM (1996) Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12:390–397
Weinstein M, Xu X, Ohyama K, Deng CX (1998) FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125:3615–3623
Bates CM (2011) Role of fibroblast growth factor receptor signaling in kidney development. Am J Physiol Renal Physiol 301:F245–F251
Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM (2004) Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276:403–415
Sims-Lucas S, Cullen-McEwen L, Eswarakumar VP, Hains D, Kish K, Becknell B, Zhang J, Bertram JF, Wang F, Bates CM (2009) Deletion of Frs2α from the ureteric epithelium causes renal hypoplasia. Am J Physiol Renal Physiol 297:F1208–F1219
Eswarakumar VP, Ozcan F, Lew ED, Bae JH, Tome F, Booth CJ, Adams DJ, Lax I, Schlessinger J (2006) Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc Natl Acad Sci USA 103:18603–18608
Sims-Lucas S, Cusack B, Eswarakumar VP, Zhang J, Wang F, Bates CM (2011) Independent roles of Fgfr2 and Frs2α in ureteric epithelium. Development 138:1275–1280
Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM (2006) Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291:325–339
Hains D, Sims-Lucas S, Kish K, Saha M, McHugh K, Bates CM (2008) Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr Res 64:592–598
Sims-Lucas S, Cusack B, Baust J, Eswarakumar VP, Masatoshi H, Takeuchi A, Bates CM (2011) Fgfr1 and the IIIc isoform of Fgfr2 play critical roles in the metanephric mesenchyme mediating early inductive events in kidney development. Dev Dyn 240:240–249
Baertschi S, Zhuang L, Trueb B (2007) Mice with a targeted disruption of the FgfrL1 gene die at birth due to alterations in the diaphragm. FEBS J 274:6241–6253
Gerber SD, Steinberg F, Beyeler M, Villiger PM, Trueb B (2009) The murine FgfrL1 receptor is essential for the development of the metanephric kidney. Dev Biol 335:106–119
Gerber SD, Amann R, Wyder S, Trueb B (2012) Comparison of the gene expression profiles from normal and FgfrL1 deficient mouse kidneys reveals downstream targets of FgfrL1 signaling. PLoS ONE 7:e33457
Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS (2008) Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell 15:781–791
Catela C, Bilbao-Cortes D, Slonimsky E, Kratsios P, Rosenthal N, Te Welscher P (2009) Multiple congenital malformations of Wolf-Hirschhorn syndrome are recapitulated in FgfrL1 null mice. Dis Model Mech 2:283–294
Potter EL (1962) Pathology of the fetus and infant, 2nd edn. Year Book Medical Publishers, Chicago
Gubler MC, Antignac C (2010) Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int 77:400–406
Smith NP, Losty PD, Connell MG, Mayer U, Jesudason EC (2006) Abnormal lung development precedes oligohydramnios in a transgenic murine model of renal dysgenesis. J Urol 175:783–786
Anderson SJ, Brennan J, de Sauvage FJ, Ding Z, Edwards J, Fikes NA, Huang W, Ouyang W, Rangel C, Sangha M, Shi Z–Z, Sparks MJ, Trackey J, Vetter M, Wang C-Y, Woodings J (2007) Novel gene disruptions, compositions and methods relating thereto. United States Patent Application 20070292438
Katoh M, Katoh M (2006) Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol Ther 5:1059–1064
Stulberg MJ, Lin A, Zhao H, Holley SA (2012) Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud. Dev Biol 369:298–307
Kobayashi A, Kwan KM, Carroll TJ, McMahon AP, Mendelsohn CL, Behringer RR (2005) Distinct and sequential tissue-specific activities of the LIM-class homeobox gene Lim1 for tubular morphogenesis during kidney development. Development 132:2809–2823
Hayashi S, Itoh M, Taira S, Agata K, Taira M (2004) Expression patterns of Xenopus FGF receptor-like 1/nou-darake in early Xenopus development resemble those of planarian nou-darake and Xenopus FGF8. Dev Dyn 230:700–707
Martinez-Morales JR, Del Bene F, Nica G, Hammerschmidt M, Bovolenta P, Wittbrodt J (2005) Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev Cell 8:565–574
Chi CL, Martinez S, Wurst W, Martin GR (2003) The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 130:2633–2644
Cebria F, Kobayashi C, Umesono Y, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Sanchez Alvarado A, Agata K (2002) FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature 419:620–624
Acknowledgments
Research in the laboratory of the authors was supported by grants from the Swiss National Science Foundation (31003A-143350), from the Helmut Horten Foundation and from the Swiss Foundation for Research on Muscular Diseases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trueb, B., Amann, R. & Gerber, S.D. Role of FGFRL1 and other FGF signaling proteins in early kidney development. Cell. Mol. Life Sci. 70, 2505–2518 (2013). https://doi.org/10.1007/s00018-012-1189-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1189-9