Abstract
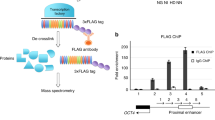
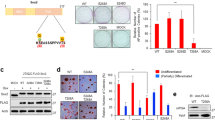
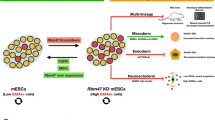
Argonaute 2 (Ago2) is a pivotal regulator of cell fate in adult stem cells. Its expression is significantly downregulated in late passages of cells, concomitant with a prominent increase in Ago2 cytosolic localization in single cells. Nuclear localization of Ago2 is crucial for the survival, proliferation, and differentiation of hATSCs (human adipose tissue-derived stem cells), mediated by the specific binding of the regulatory regions of functional genes, which positively or negatively altered gene expression. Ago2 targets genes that control stemness, reactive oxygen species scavenging, and microRNA expression, all of which are crucial for hATSC survival and self-renewal. Ago2 promotes cell proliferation and self-renewal by activating the expression of octamer-binding transcription factor 4 (Oct4). We confirmed the direct regulation of Oct4 activity by Ago2, as indicated by the results of the ChIP analysis. Methyl-CpG-binding protein 6 (MBD6) was detected as an Oct4 regulatory gene. As predicted, knockdown of MBD6 expression attenuated cell proliferation and eventually induced cell death. We hypothesized that MBD6 functions downstream of Oct4 in the regulation of stemness-related genes, cell proliferation, self-renewal activity, and survival. MBD6 also promoted cell transdifferentiation into neural and endodermal β-cells while significantly attenuating differentiation into the mesodermal lineage. We demonstrate that MBD6 is regulated by Ago2 via an interaction with Oct4, which alters self-renewal and gene expression in hATSCs. MBD6 was promoted cell proliferation through a novel set of signal mediators that may influence differentiation by repressing MBD2 and MBD3, which are possibly recruited by germ cell nuclear factor (GCNF).










Similar content being viewed by others
Abbreviations
- Ago2:
-
Argonaute2
- BRCA2:
-
Breast cancer type 2 susceptibility protein
- BrdU:
-
5-bromo-2′-deoxyuridine
- CDK:
-
Cyclin-dependent kinases
- CFU:
-
Colony forming unit
- CM-Dil:
-
Chloromethyl-benzamidodialkylcarbocyanine
- DCAF-DA:
-
1′, 7′-Dichlodihydrofluorescein diacetate
- FOXG1:
-
Forkhead box protein G1
- GFAP:
-
Glial fibrillary acidic protein
- hATSC:
-
human Adipose Tissue-derived Stem Cell
- JNK:
-
c-Jun N-terminal kinases
- KLF4:
-
Krueppel-like factor 4
- MAP2:
-
Microtubule-associated protein 2
- MBD:
-
Methyl-CpG-binding protein
- NF160:
-
Neurofilament 160
- Oct4:
-
Octamer-binding transcription factor 4
- PPARγ:
-
Peroxisome proliferator-activated receptor gamma
- RISC:
-
RNA-induced silencing complex
- ROS:
-
Reactive Oxygen Species
- Runx3:
-
Runt-related transcription factor 3
- SEPN1:
-
Selenoprotein N1
- Sox2:
-
SRY (sex determing region Y)-box 2
- STAT3:
-
Signal transducer and activator of transcription 3
- Tuj:
-
beta III tubulin
- USP44:
-
Upiquitin carboxyl-terminal hydrolase 44
References
Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ (2005) Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci USA 102:12135–12140
Jang JH, Jung JS, Im YB, Kang KS, Choi JI, Kang SK (2012) Crucial role of nuclear Ago2 for hUCB-MSCs differentiation and self-renewal via stemness control. Antioxid Redox Signal 16:95–111
Tan GS, Garchow BG, Liu X, Yeung J, Morris JPt, Cuellar TL, McManus MT, Kiriakidou M (2009) Expanded RNA-binding activities of mammalian Argonaute 2. Nucleic Acids Res 37:7533–7545
Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP (2005) The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science 310:1817–1821
Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D (2011) Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8:499–510
Chambers I, Smith A (2004) Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23:7150–7160
Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS (2003) Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol 183:355–366
Kelly DP (2011) Cell biology: ageing theories unified. Nature 470:342–343
Kim JH, Lee MR, Jee MK, Kang SK (2008) Selenium induces improvement of stem cell behaviors in human adipose-tissue stromal cells via SAPK/JNK and stemness acting signals. Stem Cells 26:2724–2734
Mattson MP (2011) A reaction to mitochondria in action. Cell Res 21:279–282
Kim BS, Jung JS, Jang JH, Kang KS, Kang SK (2011) Nuclear Argonaute 2 regulates adipose tissue-derived stem cell survival through direct control of miR10b and selenoprotein N1 expression. Aging Cell 10:277–291
Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T (2004) Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15:185–197
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Yamanaka S, Takahashi K (2006) Induction of pluripotent stem cells from mouse fibroblast cultures. Tanpakushitsu Kakusan Koso 51:2346–2351
Davey C, Pennings S, Allan J (1997) CpG methylation remodels chromatin structure in vitro. J Mol Biol 267:276–288
Kass SU, Pruss D, Wolffe AP (1997) How does DNA methylation repress transcription? Trends Genet 13:444–449
Ng HH, Bird A (1999) DNA methylation and chromatin modification. Curr Opin Genet Dev 9:158–163
Razin A (1998) CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J 17:4905–4908
Meehan RR, Lewis JD, McKay S, Kleiner EL, Bird AP (1989) Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell 58:499–507
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, Wolffe AP (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19:187–191
Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386–389
Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, Tempst P, Reinberg D, Bird A (1999) MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet 23:58–61
Cross SH, Meehan RR, Nan X, Bird A (1997) A component of the transcriptional repressor MeCP1 shares a motif with DNA methyltransferase and HRX proteins. Nat Genet 16:256–259
Hendrich B, Bird A (1998) Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 18:6538–6547
Ohki I, Shimotake N, Fujita N, Nakao M, Shirakawa M (1999) Solution structure of the methyl-CpG-binding domain of the methylation-dependent transcriptional repressor MBD1. EMBO J 18:6653–6661
Wakefield RI, Smith BO, Nan X, Free A, Soteriou A, Uhrin D, Bird AP, Barlow PN (1999) The solution structure of the domain from MeCP2 that binds to methylated DNA. J Mol Biol 291:1055–1065
Wade PA, Gegonne A, Jones PL, Ballestar E, Aubry F, Wolffe AP (1999) Mi-2 complex couples DNA methylation to chromatin remodelling and histone deacetylation. Nat Genet 23:62–66
Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D (1999) Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev 13:1924–1935
Jang JH, Jung JS, Choi JI, Kang SK (2012) Nuclear Ago2/HSP60 contributes to broad spectrum of hATSCs function via Oct4 regulation. Antioxid Redox Signal 16(5):383–399
Jiang CL, Jin SG, Pfeifer GP (2004) MBD3L1 is a transcriptional repressor that interacts with methyl-CpG-binding protein 2 (MBD2) and components of the NuRD complex. J Biol Chem 279:52456–52464
Sakai H, Urano T, Ookata K, Kim MH, Hirai Y, Saito M, Nojima Y, Ishikawa F (2002) MBD3 and HDAC1, two components of the NuRD complex, are localized at Aurora-A-positive centrosomes in M phase. J Biol Chem 277:48714–48723
Zhu D, Fang J, Li Y, Zhang J (2009) Mbd3, a component of NuRD/Mi-2 complex, helps maintain pluripotency of mouse embryonic stem cells by repressing trophectoderm differentiation. PLoS ONE 4:e7684
Petronzelli F, Riccio A, Markham GD, Seeholzer SH, Stoerker J, Genuardi M, Yeung AT, Matsumoto Y, Bellacosa A (2000) Biphasic kinetics of the human DNA repair protein MED1 (MBD4), a mismatch-specific DNA N-glycosylase. J Biol Chem 275:32422–32429
Tatematsu KI, Yamazaki T, Ishikawa F (2000) MBD2–MBD3 complex binds to hemi-methylated DNA and forms a complex containing DNMT1 at the replication foci in late S phase. Genes Cells 5:677–688
Zhang Y, van Deursen J, Galardy PJ (2011) Overexpression of ubiquitin specific protease 44 (USP44) induces chromosomal instability and is frequently observed in human T-cell leukemia. PLoS ONE 6:e23389
Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig U, Blasco MA, Jonkers J, Tarsounas M (2010) BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol 17:1461–1469
Laget S, Joulie M, Le Masson F, Sasai N, Christians E, Pradhan S, Roberts RJ, Defossez PA (2010) The human proteins MBD5 and MBD6 associate with heterochromatin but they do not bind methylated DNA. PLoS ONE 5:e11982
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST, 2010-0020265).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin Sun Jung and Min Ki Jee contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, J.S., Jee, M.K., Cho, H.T. et al. MBD6 is a direct target of Oct4 and controls the stemness and differentiation of adipose tissue-derived stem cells. Cell. Mol. Life Sci. 70, 711–728 (2013). https://doi.org/10.1007/s00018-012-1157-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1157-4