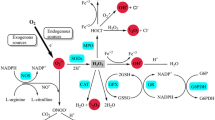
Abstract
Among the pathogenic mechanisms underlying central nervous system (CNS) diseases, oxidative stress is almost invariably described. For this reason, numerous attempts have been made to decrease reactive oxygen species (ROS) with the administration of antioxidants as potential therapies for CNS disorders. However, such treatments have always failed in clinical trials. Targeting specific sources of reactive oxygen species in the CNS (e.g. NOX enzymes) represents an alternative promising option. Indeed, NOX enzymes are major generators of ROS, which regulate progression of CNS disorders as diverse as amyotrophic lateral sclerosis, schizophrenia, Alzheimer disease, Parkinson disease, and stroke. On the other hand, in autoimmune demyelinating diseases, ROS generated by NOX enzymes are protective, presumably by dampening the specific immune response. In this review, we discuss the possibility of developing therapeutics targeting NADPH oxidase (NOX) enzymes for the treatment of different CNS pathologies. Specific compounds able to modulate the activation of NOX enzymes, and the consequent production of ROS, could fill the need for disease-modifying drugs for many incurable CNS pathologies.


Similar content being viewed by others
References
Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D (2008) Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9:686–696
Aliev G, Palacios HH, Walrafen B, Lipsitt AE, Obrenovich ME, Morales L (2009) Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int J Biochem Cell Biol 41:1989–2004
Ansari MA, Scheff SW (2011) NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med 51:171–178
Ashrafian H, Horowitz JD, Frenneaux MP (2007) Perhexiline. Cardiovasc Drug Rev 25:76–97
Barber SC, Shaw PJ (2010) Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med 48:629–641
Barten LJ, Allington DR, Procacci KA, Rivey MP (2010) New approaches in the management of multiple sclerosis. Drug Des Dev Ther 4:343–366
Beal MF (2002) Oxidatively modified proteins in aging and disease. Free Radic Biol Med 32:797–803
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Beebe DW, Gozal D (2002) Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 11:1–16
Beghi E, Chio A, Couratier P, Esteban J, Hardiman O, Logroscino G, Millul A, Mitchell D, Preux PM, Pupillo E, Stevic Z, Swingler R, Traynor BJ, Van den Berg LH, Veldink JH, Zoccolella S (2011) The epidemiology and treatment of ALS: focus on the heterogeneity of the disease and critical appraisal of therapeutic trials. Amyotroph Later Scler 12:1–10
Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL (2007) Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 318:1645–1647
Behrens MM, Ali SS, Dugan LL (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci 28:13957–13966
Behrens MM, Sejnowski TJ (2009) Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology 57:193–200
Bendtzen K (2010) Critical review: assessment of interferon-beta immunogenicity in multiple sclerosis. J Interfer Cytokine Res 30:759–766
Bensimon A, Simon A, Chiffaudel A, Croquette V, Heslot F, Bensimon D (1994) Alignment and sensitive detection of DNA by a moving interface. Science 265:2096–2098
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI (2008) N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64:361–368
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Borbely G, Szabadkai I, Horvath Z, Marko P, Varga Z, Breza N, Baska F, Vantus T, Huszar M, Geiszt M, Hunyady L, Buday L, Orfi L, Keri G (2010) Small-molecule inhibitors of NADPH oxidase 4. J Med Chem 53:6758–6762
Braunersreuther V, Jaquet V (2011) Reactive Oxygen Species in Myocardial Reperfusion Injury: From Physiopathology to Therapeutic Approaches. Curr Pharm Biotechnol 13:97–114
Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA (2009) NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci 12:857–863
Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM (2011) Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens 5:137–153
Bromet EJ, Fennig S (1999) Epidemiology and natural history of schizophrenia. Biol Psychiatry 46:871–881
Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ (2002) Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 122:1162–1167
Carter MD, Simms GA, Weaver DF (2010) The development of new therapeutics for Alzheimer’s disease. Clin Pharmacol Ther 88:475–486
Carter NJ, Keating GM (2010) Glatiramer acetate: a review of its use in relapsing-remitting multiple sclerosis and in delaying the onset of clinically definite multiple sclerosis. Drugs 70:1545–1577
Caslake R, Macleod A, Ives N, Stowe R, Counsell C (2009) Monoamine oxidase B inhibitors versus other dopaminergic agents in early Parkinson’s disease. Cochrane Database Syst Rev: CD006661
Chen H, Song YS, Chan PH (2009) Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab 29:1262–1272
Choi DH, Cristovao AC, Guhathakurta S, Joh T, Beal F, Kim YS (2011) NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson’s disease. Antioxid Redox Signal 16:1033–1045
Choi DK, Koppula S, Choi M, Suk K (2010) Recent developments in the inhibitors of neuroinflammation and neurodegeneration: inflammatory oxidative enzymes as a drug target. Expert Opin Ther Pat 20:1531–1546
Cleren C, Calingasan NY, Chen J, Beal MF (2005) Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem 94:995–1004
Cristovao AC, Choi DH, Baltazar G, Beal MF, Kim YS (2009) The role of NADPH oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid Redox Signal 11:2105–2118
Cui C, Chen AF, Jiang Z, Wu Q, Lin J, Wen H, Zeng J (2007) Inhibition of NAD(P)H oxidase reduces fibronectin expression in stroke-prone renovascular hypertensive rat brain. Clin Exp Pharmacol Physiol 34:304–309
de la Monte SM, Wands JR (2006) Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis 9:167–181
Dumont M, Stack C, Elipenhali C, Calingasan NY, Wille E, Beal MF (2011) Apocynin administration does not improve behavioral and neuropathological deficits in a transgenic mouse model of Alzheimer’s disease. Neurosci Lett 492:150–154
Duty S, Jenner P (2011) Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol 164:1357–1391
Engel O, Kolodziej S, Dirnagl U, Prinz V (2011) Modeling stroke in mice—middle cerebral artery occlusion with the filament model. J Vis Exp. doi: 10.3791/2423
Fabian RH, Perez-Polo JR, Kent TA (2008) Perivascular nitric oxide and superoxide in neonatal cerebral hypoxia-ischemia. Am J Physiol Heart Circ Physiol 295:H1809–H1814
Farber NB (2003) The NMDA receptor hypofunction model of psychosis. Ann NY Acad Sci 1003:119–130
Filippini G, Munari L, Incorvaia B, Ebers GC, Polman C, D’Amico R, Rice GP (2003) Interferons in relapsing remitting multiple sclerosis: a systematic review. Lancet 361:545–552
Fragoso YD, Frota ER, Lopes JS, Noal JS, Giacomo MC, Gomes S, Goncalves MV, da Gama PD, Finkelsztejn A (2010) Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin Neuropharmacol 33:312–316
Fraser PA (2011) The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med 51:967–977
Fulton DJ (2009) Nox5 and the regulation of cellular function. Antioxid Redox Signal 11:2443–2452
Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P (2011) Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg Med Chem 19:6989–6999
Gatto GJ, Ao Z, Kearse MG, Zhou M, Morales CR, Daniels E, Bradley BT, Goserud MT, Goodman KB, Douglas SA, Harpel MR, Johns DG (2012) NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J Enzyme Inhib Med Chem (in press)
Genovese T, Mazzon E, Paterniti I, Esposito E, Bramanti P, Cuzzocrea S (2011) Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res 1372:92–102
Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H (2010) A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5:981–993
Giustarini D, Dalle-Donne I, Tsikas D, Rossi R (2009) Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci 46:241–281
Gold R (2011) Oral therapies for multiple sclerosis: a review of agents in phase III development or recently approved. CNS Drugs 25:37–52
Gordon M, Gordon AS (1981) Perhexiline maleate as a cause of reversible parkinsonism and peripheral neuropathy. J Am Geriatr Soc 29:259–262
Gordon PH (2011) Amyotrophic lateral sclerosis: pathophysiology, diagnosis and management. CNS Drugs 25:1–15
Gupta RK, Chandra A, Verm AK, Kumar S (2010) Obstructive sleep apnoea: a clinical review. J Assoc Physicians India 58:438–441
Hall DJ, Han SH, Chepetan A, Inui EG, Rogers M, Dugan LL (2011) Dynamic optical imaging of metabolic and NADPH oxidase-derived superoxide in live mouse brain using fluorescence lifetime unmixing. J Cereb Blood Flow Metab 32:23–32
Halliday GM, Stevens CH (2011) Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord 26:6–17
Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schoneich C, Engelhardt JF (2008) SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest 118:659–670
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4:600–609
Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51:211–217
Hofstetter HH, Grau C, Buttmann M, Forsthuber TG, Gaupp S, Toyka KV, Gold R (2007) The PLPp-specific T-cell population promoted by pertussis toxin is characterized by high frequencies of IL-17-producing cells. Cytokine 40:35–43
Huberle A, Beyeen AD, Ockinger J, Ayturan M, Jagodic M, de Graaf KL, Fissolo N, Marta M, Olofsson P, Hultqvist M, Holmdahl R, Olsson T, Weissert R (2009) Advanced intercross line mapping suggests that ncf1 (ean6) regulates severity in an animal model of guillain-barre syndrome. J Immunol 182:4432–4438
Hughes RA, Pritchard J, Hadden RD (2011) Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain Barre syndrome. Cochrane Database Syst Rev: CD008630
Hui-guo L, Kui L, Yan-ning Z, Yong-jian X (2010) Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med 11:205–212
Hultqvist M, Olofsson P, Gelderman KA, Holmberg J, Holmdahl R (2006) A new arthritis therapy with oxidative burst inducers. PLoS Med 3:e348
Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R (2004) Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA 101:12646–12651
Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R (2009) The protective role of ROS in autoimmune disease. Trends Immunol 30:201–208
Hutchinson M (2010) Natalizumab therapy of multiple sclerosis. J Interfer Cytokine Res 30:787–789
Infante-Duarte C, Waiczies S, Wuerfel J, Zipp F (2008) New developments in understanding and treating neuroinflammation. J Mol Med 86:975–985
Jackman KA, Miller AA, De Silva TM, Crack PJ, Drummond GR, Sobey CG (2009) Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol 156:680–688
Jaquet V, Bedard K (2009) Editorial: Genetic mapping–the path of discovery for novel functions of the NOX NADPH oxidases. J Leukoc Biol 86:461–463
Jaquet V, Marcoux J, Forest E, Leidal KG, McCormick S, Westermaier Y, Perozzo R, Plastre O, Fioraso-Cartier L, Diebold B, Scapozza L, Nauseef WM, Fieschi F, Krause KH, Bedard K (2011) NOX NADPH oxidase isoforms are inhibited by celastrol with a dual mode of action. Br J Pharmacol 164:507–520
Jaquet V, Scapozza L, Clark R, Krause KH, Lambeth JD (2009) Small Molecule NOX Inhibitors: ROS-generating NADPH Oxidases as Therapeutic Targets. Antioxid Redox Signal 11:2535–2552
Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP (2007) NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38:3000–3006
Kane JM, Correll CU (2010) Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry 71:1115–1124
Kannaiyan R, Shanmugam MK, Sethi G (2011) Molecular targets of celastrol derived from Thunder of God Vine: potential role in the treatment of inflammatory disorders and cancer. Cancer Lett 303:9–20
Kelly KA, Li X, Tan Z, VanGilder RL, Rosen CL, Huber JD (2009) NOX2 inhibition with apocynin worsens stroke outcome in aged rats. Brain Res 1292:165–172
Kiaei M, Kipiani K, Petri S, Chen J, Calingasan NY, Beal MF (2005) Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener Dis 2:246–254
Kim JA, Neupane GP, Lee ES, Jeong BS, Park BC, Thapa P (2011) NADPH oxidase inhibitors: a patent review. Expert Opin Ther Pat 21:1148–1158
Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM (2006) A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci 26:1604–1615
Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, Schmidt HH (2010) Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8(9). (pii: e1000479)
Kurz A, Perneczky R (2009) Neurobiology of cognitive disorders. Curr Opin Psychiatry 22:546–551
Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V (1996) Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347:1425–1431
Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, Page P (2010) First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53:7715–7730
Lambeth JD, Krause KH, Clark RA (2008) NOX enzymes as novel targets for drug development. Semin Immunopathol 30:339–363
Lee HP, Zhu X, Casadesus G, Castellani RJ, Nunomura A, Smith MA, Lee HG, Perry G (2010) Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev Neurother 10:1201–1208
Leng A, Feldon J, Ferger B (2004) Long-term social isolation and medial prefrontal cortex: dopaminergic and cholinergic neurotransmission. Pharmacol Biochem Behav 77:371–379
Leto TL, Morand S, Hurt D, Ueyama T (2009) Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11:2607–2619
Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML (2010) Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity. Brain 133:808–821
Lewis DA, Hashimoto T, Volk DW (2005) Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324
Lewis DA, Lieberman JA (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334
Liebeskind DS, Kasner SE (2001) Neuroprotection for ischaemic stroke: an unattainable goal? CNS Drugs 15:165–174
Lincecum JM, Vieira FG, Wang MZ, Thompson K, De Zutter GS, Kidd J, Moreno A, Sanchez R, Carrion IJ, Levine BA, Al-Nakhala BM, Sullivan SM, Gill A, Perrin S (2010) From transcriptome analysis to therapeutic anti-CD40L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet 42:392–399
Liu HG, Liu K, Zhou YN, Xu YJ (2009) Relationship between reduced nicotinamide adenine dinucleotide phosphate oxidase subunit p22phox gene polymorphism and obstructive sleep apnea-hypopnea syndrome in the Chinese Han population. Chin Med J (Engl) 122:1369–1374
Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Cetin O, Miyake M, Liu KJ (2008) Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem 107:1196–1205
Lo A (2008) Advancement of therapies for neuroprotection in multiple sclerosis. Expert Rev Neurother 8:1355–1366
Lopate G, Pestronk A (2011) Inflammatory demyelinating neuropathies. Curr Treat Options Neurol 13:131–142
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7:354–365
Lull ME, Levesque S, Surace MJ, Block ML (2011) Chronic apocynin treatment attenuates beta amyloid plaque size and microglial number in hAPP(751)(SL) mice. PLoS ONE 6:e20153
Maldonado PD, Molina-Jijon E, Villeda-Hernandez J, Galvan-Arzate S, Santamaria A, Pedraza-Chaverri J (2010) NAD(P)H oxidase contributes to neurotoxicity in an excitotoxic/prooxidant model of Huntington’s disease in rats: protective role of apocynin. J Neurosci Res 88:620–629
Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF (2007) Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest 117:2913–2919
Marriott JJ, Miyasaki JM, Gronseth G, O’Connor PW (2010) Evidence Report: the efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 74:1463–1470
Marsh JD, Keyrouz SG (2010) Stroke prevention and treatment. J Am Coll Cardiol 56:683–691
Martinelli V, Radaelli M, Straffi L, Rodegher M, Comi G (2009) Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci 30(Suppl 2):S167–S170
Masood A, Nadeem A, Mustafa SJ, O’Donnell JM (2008) Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther 326:369–379
Massaad CA, Amin SK, Hu L, Mei Y, Klann E, Pautler RG (2010) Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer’s disease. PLoS ONE 5:e10561
Melnikova I (2007) Therapies for Alzheimer’s disease. Nat Rev Drug Discov 6:341–342
Miana-Mena FJ, Piedrafita E, Gonzalez-Mingot C, Larrode P, Munoz MJ, Martinez-Ballarin E, Reiter RJ, Osta R, Garcia JJ (2011) Levels of membrane fluidity in the spinal cord and the brain in an animal model of amyotrophic lateral sclerosis. J Bioenerg Biomembr 43:181–186
Miller RG, Mitchell JD, Lyon M, Moore DH (2003) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord 4:191–206
Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, Mahata J, Kilty M, Bednarz K, Bell D, Gordon PH, Hornig M, Mehrazin M, Naini A, Flint Beal M, Factor-Litvak P (2008) Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler 9:177–183
Mohamed T, Rao PN (2011) Alzheimer’s disease: emerging trends in small molecule therapies. Curr Med Chem 18:4299–4320
Mossberg N, Andersen O, Nilsson S, Dahlgren C, Hellstrand K, Lindh M, Svedhem A, Bergstrom T, Movitz C (2007) Oxygen radical production and severity of the Guillain–Barre syndrome. J Neuroimmunol 192:186–191
Mossberg N, Andersen O, Nordin M, Nilsson S, Svedhem A, Bergstrom T, Hellstrand K, Movitz C (2010) Leukocyte oxygen radical production determines disease severity in the recurrent Guillain–Barre syndrome. J Inflamm (Lond) 7:40
Mossberg N, Movitz C, Hellstrand K, Bergstrom T, Nilsson S, Andersen O (2009) Oxygen radical production in leukocytes and disease severity in multiple sclerosis. J Neuroimmunol 213:131–134
Murotomi K, Takagi N, Takeo S, Tanonaka K (2011) NADPH oxidase-mediated oxidative damage to proteins in the postsynaptic density after transient cerebral ischemia and reperfusion. Mol Cell Neurosci 46:681–688
Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D (2011) Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS ONE 6:e19847
Nakanishi N, Tu S, Shin Y, Cui J, Kurokawa T, Zhang D, Vincent Chen H-S, Tong G, Lipton SA (2009) Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci 29:5260–526
Ohlow MJ, Moosmann B (2011) Phenothiazine: the seven lives of pharmacology’s first lead structure. Drug Discov Today 16:119–131
Ohye H, Sugawara M (2010) Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood) 235:424–433
Olofsson P, Holmberg J, Tordsson J, Lu S, Akerstrom B, Holmdahl R (2003) Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet 33:25–32
Palmer AM, Stephenson FA (2005) CNS drug discovery: challenges and solutions. Drug News Perspect 18:51–57
Paris D, Ganey NJ, Laporte V, Patel NS, Beaulieu-Abdelahad D, Bachmeier C, March A, Ait-Ghezala G, Mullan MJ (2010) Reduction of beta-amyloid pathology by celastrol in a transgenic mouse model of Alzheimer’s disease. J Neuroinflamm 7:17
Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C (2008) Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105:1347–1352
Patten DA, Germain M, Kelly MA, Slack RS (2010) Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis 20(Suppl 2):S357–S367
Perez-Lloret S, Rascol O (2010) Dopamine receptor agonists for the treatment of early or advanced Parkinson’s disease. CNS Drugs 24:941–968
Pestana RR, Kinjo ER, Hernandes MS, Britto LR (2010) Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci Lett 484:187–191
Peters EA, Hiltermann JT, Stolk J (2001) Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31:1442–1447
Polymenidou M, Cleveland DW (2011) The seeds of neurodegeneration: prion-like spreading in ALS. Cell 147:498–508
Pozzilli C, Prosperini L, Borriello G (2010) Treating multiple sclerosis with fingolimod or intramuscular interferon. Expert Opin Pharmacother 11:1957–1960
Pratico D (2010) The neurobiology of isoprostanes and Alzheimer’s disease. Biochim Biophys Acta 1801:930–933
Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA (2007) Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis 25:392–400
Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, Krause KH (2006) A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging 27:1577–1587
Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279:1415–1421
Rappold PM, Cui M, Chesser AS, Tibbett J, Grima JC, Duan L, Sen N, Javitch JA, Tieu K (2011) Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci USA 108:20766–20771
Reddy R (2011) Antioxidant Therapeutics for Schizophrenia. Antioxid Redox Signal 15:2047–2055
Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ (2003) Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348:1333–1341
Said G (1978) Perhexiline neuropathy: a clinicopathological study. Ann Neurol 3:259–266
Sanchez-Moreno C, Jimenez-Escrig A, Martin A (2009) Stroke: roles of B vitamins, homocysteine and antioxidants. Nutr Res Rev 22:49–67
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116:1744–1754
Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause KH (2009) Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry 66:384–392
Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F (2000) Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 162:566–570
Sedeek M, Callera G, Montezano A, Gutsol A, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hebert RL (2010) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Renal Physiol 299:F1348–F1358
Seet RC, Lee CY, Lim EC, Tan JJ, Quek AM, Chong WL, Looi WF, Huang SH, Wang H, Chan YH, Halliwell B (2010) Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radic Biol Med 48:560–566
Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A, Chiba S, Perry G, Smith MA, Fujimoto S (2000) Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun 273:5–9
Simonyi A, He Y, Sheng W, Sun AY, Wood WG, Weisman GA, Sun GY (2010) Targeting NADPH oxidase and phospholipases A2 in Alzheimer’s disease. Mol Neurobiol 41:73–86
Sorce S, Krause KH (2009) NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11:2481–2504
Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, Krause KH (2010) The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 30:11317–11325
Stefanska J, Pawliczak R (2008) Apocynin: molecular aptitudes. Mediat Inflamm 2008:106507
Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ (1994) Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11:95–102
Sudeshna G, Parimal K (2010) Multiple non-psychiatric effects of phenothiazines: a review. Eur J Pharmacol 648:6–14
Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA (2007) Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 117:910–918
Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA (2008) Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol 64:654–663
Tang LL, Ye K, Yang XF, Zheng JS (2007) Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res 35:517–522
Tang XN, Cairns B, Cairns N, Yenari MA (2008) Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 154:556–562
Titova E, Ostrowski RP, Sowers LC, Zhang JH, Tang J (2007) Effects of apocynin and ethanol on intracerebral haemorrhage-induced brain injury in rats. Clin Exp Pharmacol Physiol 34:845–850
Trumbull KA, McAllister D, Gandelman MM, Fung WY, Lew T, Brennan L, Lopez N, Morre J, Kalyanaraman B, Beckman JS (2012) Diapocynin and apocynin administration fails to significantly extend survival in G93A SOD1 ALS mice. Neurobiol Dis 45:137–144
van Horssen J, Witte ME, Schreibelt G, de Vries HE (2011) Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta 1812:141–150
Vendrov AE, Madamanchi NR, Niu XL, Molnar KC, Runge M, Szyndralewiez C, Page P, Runge MS (2010) NADPH oxidases regulate CD44 and hyaluronic acid expression in thrombin-treated vascular smooth muscle cells and in atherosclerosis. J Biol Chem 285:26545–26557
Violi F, Sanguigni V, Carnevale R, Plebani A, Rossi P, Finocchi A, Pignata C, De Mattia D, Martire B, Pietrogrande MC, Martino S, Gambineri E, Soresina AR, Pignatelli P, Martino F, Basili S, Loffredo L (2009) Hereditary deficiency of gp91(phox) is associated with enhanced arterial dilatation: results of a multicenter study. Circulation 120:1616–1622
Violi F, Sanguigni V, Loffredo L, Carnevale R, Buchetti B, Finocchi A, Tesauro M, Rossi P, Pignatelli P (2006) Nox2 is determinant for ischemia-induced oxidative stress and arterial vasodilatation: a pilot study in patients with hereditary Nox2 deficiency. Arterioscler Thromb Vasc Biol 26:e131–e132
Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR (1997) Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 28:2252–2258
Wan Y, Xu J, Meng F, Bao Y, Ge Y, Lobo N, Vizcaychipi MP, Zhang D, Gentleman SM, Maze M, Ma D (2010) Cognitive decline following major surgery is associated with gliosis, beta-amyloid accumulation, and tau phosphorylation in old mice. Crit Care Med 38:2190–2198
Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY (2008) Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine 15:496–503
Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY (2006) Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res 1090:182–189
Wang Y, Zhang SX, Gozal D (2010) Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol 174:307–316
Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, Wingler K (2010) Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161:885–898
Wipfler P, Harrer A, Pilz G, Oppermann K, Trinka E, Kraus J (2011) Recent developments in approved and oral multiple sclerosis treatment and an update on future treatment options. Drug Discov Today 16:8–21
Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S (2006) The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA 103:12132–12137
Xian NT, Zhen Z, Rona GG, Midori AY (2011) Significance of marrow derived NADPH oxidase in experimental ischemic stroke. Ann Neurol 70:606–615
Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, Fukuda M, Matsuba S, Ogawa H, Kim-Mitsuyama S (2008) Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke 39:3049–3056
Yoshioka H, Niizuma K, Katsu M, Okami N, Sakata H, Kim GS, Narasimhan P, Chan PH (2011) NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J Cereb Blood Flow Metab 31:868–880
Zamvil SS, Steinman L (1990) The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol 8:579–621
Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E, Veasey SC (2005) NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 172:921–929
Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF, Hong JS, Liu B (2004) Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J 18:589–591
Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H (2010) Alcohol-induced exacerbation of ischemic brain injury: role of NAD(P)H oxidase. Alcohol Clin Exp Res 34:1948–1955
Zheng JS, Zhan RY, Zheng SS, Zhou YQ, Tong Y, Wan S (2005) Inhibition of NADPH oxidase attenuates vasospasm after experimental subarachnoid hemorrhage in rats. Stroke 36:1059–1064
Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, Veasey SC (2007) Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci 27:10060–10071
Zia MT, Csiszar A, Labinskyy N, Hu F, Vinukonda G, LaGamma EF, Ungvari Z, Ballabh P (2009) Oxidative-nitrosative stress in a rabbit pup model of germinal matrix hemorrhage: role of NAD(P)H oxidase. Stroke 40:2191–2198
Acknowledgments
We are grateful to Dr Karen Bedard and Dr Freddy Heitz for critical reading of the manuscript and to all the members of the NEURINOX consortium for their input in the elaboration of the concepts described in this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorce, S., Krause, KH. & Jaquet, V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell. Mol. Life Sci. 69, 2387–2407 (2012). https://doi.org/10.1007/s00018-012-1014-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1014-5