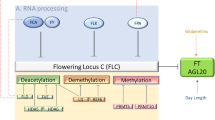
Abstract
Plants undergo a major physiological change as they transition from vegetative growth to reproductive development. This transition is a result of responses to various endogenous and exogenous signals that later integrate to result in flowering. Five genetically defined pathways have been identified that control flowering. The vernalization pathway refers to the acceleration of flowering on exposure to a long period of cold. The photoperiod pathway refers to regulation of flowering in response to day length and quality of light perceived. The gibberellin pathway refers to the requirement of gibberellic acid for normal flowering patterns. The autonomous pathway refers to endogenous regulators that are independent of the photoperiod and gibberellin pathways. Most recently, an endogenous pathway that adds plant age to the control of flowering time has been described. The molecular mechanisms of these pathways have been studied extensively in Arabidopsis thaliana and several other flowering plants.



Similar content being viewed by others
Abbreviations
- GA:
-
Gibberellic acid
- SD:
-
Short day
- LD:
-
Long day
References
Kobayashi Y, Weigel D (2007) Move on up, it’s time for change—mobile signals controlling photoperiod-dependent flowering. Genes Dev 21:2371–2384
Pittendrigh CS (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 25:159–184
Pittendrigh C (1972) Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci USA 69:2734–2737
Knott J (1934) Effect of a localized photoperiod on spinach. Proc Soc Hortic Sci 31:152–154
Evans LT (1971) Flower induction and the florigen concept. Annu Rev Plant Physiol 22:365–394
Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59:573–594
King RW, Evans LT, Wardlaw IF (1968) Translocation of the floral stimulus in Pharbitis nil in relation to that of assimilates. Z Pflanzenphysiol 59:377–388
King RW, Zeevaart JA (1973) Floral stimulus movement in perilla and flower inhibition caused by noninduced leaves. Plant Physiol 51(4):727–738
Smith H, Whitelam GC (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20(6):840–844. doi:10.1046/j.1365-3040.1997.d01-104.x
Tsuchiya T, Ishiguri Y (1981) Role of the quality of light on the photoperiodic flowering response in four latitudinal ecotypes of Chenopodium rubrum. Plant Cell Physiol 22:525–532
Gassner G (1918) Beiträge zur physiologischen Charakteristik sommer- und winterannueller Gewächse, insbesondere der Getreidepflanzen. Z Bot 10:417–480
Purvis ON, Gregory FG (1952) The reversibility by high temperature of the vernalised condition in Petkus winter rye. Ann Bot 16:1–21
Wang JY (1960) A critique of the heat unit approach to plant-response studies. Ecology 41(4):785–790
Samach A, Wigge P (2005) Ambient temperature perception in plants. Curr Opin Plant Biol 8:483–486
Lang A (1957) The effect of gibberellin upon flower formation. Proc Natl Acad Sci USA 43:709–717
Lang A (1960) Gibberellin-like substances in photoinduced and vegetative Hyoscyamus plants. Planta 54:498–504
Langridge J (1957) Effect of day-length and gibberellic acid on the flowering of Arabidopsis. Nature 180:36–37
Chandler J, Dean C (1994) Factors influencing the vernalization response and flowering time of late flowering mutants of Arabidopsis thaliana (L.) Heynh. J Exp Bot 45:1279–1288
Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P (1993) Physiological signals that induce flowering. Plant Cell 5:1147–1155
Bodson M, Outlaw WH (1985) Elevation in the sucrose content of the shoot apical meristem of Sinapis alba at floral evocation. Plant Physiol 79(2):420–424
Laibach F (1943) Arabidopsis thaliana (L.) Heynh. als Objekt für genetische und entwicklungsphysiologische Untersuchungen. Bot Arch 44:439–455
Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55:141–172. doi:10.1146/annurev.arplant.55.031903.141605
Lempe J, Balasubramanian S, Sureshkumar S, Singh A, Schmid M, Weigel D (2005) Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet 1:109–118
Sung S, Amasino RM (2004) Vernalization and epigenetics: how plants remember winter. Curr Opin Plant Biol 7(1):4–10
Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C (2000) Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290:344–347
Michaels S, Amasino R (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11:949–956
Rédei GP (1962) Supervital mutants of Arabidopsis. Genetics 47:443–460
Koornneef M, Hanhart C, van der Veen J (1991) A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet 229:57–66
Lariguet P, Dunand C (2005) Plant photoreceptors: phylogenetic overview. J Mol Evol 61:559–569
Li Q, Yang H (2007) Cryptochrome signaling in plants. Photochem Photobiol 83:94–101
Quail P, Boylan M, Parks B, Short T, Xu Y, Wagner D (1995) Phytochromes: photosensory perception and signal transduction. Science 268:675–680
Tóth R, Kevei E, Hall A, Millar A, Nagy F, Kozma-Bognar L (2001) Circadian clock-regulated expression of phytochrome and cryptochrome genes in Arabidopsis. Plant Physiol 127:1607–1616
Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80:847–857
An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Píniro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131:3615–3626
Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410:1116–1120
Fornara F, Panigrahi K, Gissot L, Sauerbrunn N, Rühl M, Jarillo J, Coupland G (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17:75–86
Imaizumi T, Schultz T, Harmon F, Ho L, Kay S (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309:293–297
Sawa M, Nusinow D, Kay S, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318:261–265
Imaizumi T, Tran H, Swartz T, Briggs W, Kay S (2003) FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426:302–306
Liu L, Zhang Y, Li Q, Sang Y, Mao J, Lian H, Wang L, Yang H (2008) COP1-mediated ubiquitination of CONSTANS is implicated in cryptochrome regulation of flowering in Arabidopsis. Plant Cell 20:292–306
Hoecker U, Quail P (2001) The phytochrome A-specific signaling intermediate SPA1 interacts directly with COP1, a constitutive repressor of light signaling in Arabidopsis. J Biol Chem 276:38173–38178
Laubinger S, Marchal V, Le Gourrierec J, Gentilhomme J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, Hoecker U (2006) Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 133:3213–3222
Laubinger S, Hoecker U (2003) The SPA1-like proteins SPA3 and SPA4 repress photomorphogenesis in the light. Plant J 35:373–385
Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303:1003–1006
Jang S, Marchal V, Panigrahi K, Wenkel S, Soppe W, Deng X, Valverde F, Coupland G (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27:1277–1288
Morris K, Thornber S, Codrai L, Richardson C, Craig A, Sadanandom A, Thomas B, Jackson S (2010) DAY NEUTRAL FLOWERING represses CONSTANS to prevent Arabidopsis flowering early in short days. Plant Cell 22(4):1118–1128. doi:10.1105/tpc.109.066605
Ayre BG, Turgeon R (2004) Graft transmission of a floral stimulant derived from CONSTANS. Plant Physiol 135(4):2271–2278. doi:10.1104/pp.104.040592
Kardailsky I, Shukla V, Ahn J, Dagenais N, Christensen S, Nguyen J, Chory J, Harrison M, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286:1962–1965
Weigel D, Ahn JH, Blazquez MA, Borevitz JO, Christensen SK, Fankhauser C, Ferrandiz C, Kardailsky I, Malancharuvil EJ, Neff MM, Nguyen JT, Sato S, Wang ZY, Xia Y, Dixon RA, Harrison MJ, Lamb CJ, Yanofsky MF, Chory J (2000) Activation tagging in Arabidopsis. Plant Physiol 122(4):1003–1013
Kaya H, Sato S, Tabata S, Kobayashi Y, Iwabuchi M, Araki T (2000) Hosoba toge toge, a syndrome caused by a large chromosomal deletion associated with a T-DNA insertion in Arabidopsis. Plant Cell Physiol 41(9):1055–1066
Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286:1960–1962
Ahn J, Miller D, Winter V, Banfield M, Lee J, Yoo S, Henz S, Brady R, Weigel D (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25:605–614
Harmer S, Hogenesch J, Straume M, Chang H, Han B, Zhu T, Wang X, Kreps J, Kay S (2000) Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290:2110–2113
Takada S, Goto K (2003) Terminal flower2, an Arabidopsis homolog of heterochromatin protein1, counteracts the activation of flowering locus T by constans in the vascular tissues of leaves to regulate flowering time. Plant Cell 15(12):2856–2865. doi:10.1105/tpc.016345
Farrona S, Coupland G, Turck F (2008) The impact of chromatin regulation on the floral transition. Semin Cell Dev Biol 19(6):560–573. doi:10.1016/j.semcdb.2008.07.015
Exner V, Aichinger E, Shu H, Wildhaber T, Alfarano P, Caflisch A, Gruissem W, Kohler C, Hennig L (2009) The chromodomain of LIKE HETEROCHROMATIN PROTEIN 1 is essential for H3K27me3 binding and function during Arabidopsis development. PLoS One 4(4):e5335. doi:10.1371/journal.pone.0005335
Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V (2007) Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet 3(6):e86. doi:10.1371/journal.pgen.0030086
Zhang X, Clarenz O, Cokus S, Bernatavichute YV, Pellegrini M, Goodrich J, Jacobsen SE (2007) Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol 5(5):e129. doi:10.1371/journal.pbio.0050129
Köhler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W (2003) Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J 22(18):4804–4814. doi:10.1093/emboj/cdg444
Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J (2006) Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J 25(19):4638–4649. doi:10.1038/sj.emboj.7601311
Yoshida N, Yanai Y, Chen L, Kato Y, Hiratsuka J, Miwa T, Sung ZR, Takahashi S (2001) EMBRYONIC FLOWER2, a novel polycomb group protein homolog, mediates shoot development and flowering in Arabidopsis. Plant Cell 13(11):2471–2481
Jiang D, Wang Y, He Y (2008) Repression of FLOWERING LOCUS C and FLOWERING LOCUS T by the Arabidopsis Polycomb repressive complex 2 components. PLoS One 3(10):e3404. doi:10.1371/journal.pone.0003404
Hennig L, Derkacheva M (2009) Diversity of Polycomb group complexes in plants: same rules, different players? Trends Genet 25(9):414–423. doi:10.1016/j.tig.2009.07.002
Adrian J, Farrona S, Reimer J, Albani M, Coupland G, Turck F (2010) cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell 22(5):1425–1440
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol 46(8):1175–1189. doi:10.1093/pcp/pci151
Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol 139(2):770–778. doi:10.1104/pp.105.066928
Wigge P, Kim M, Jaeger K, Busch W, Schmid M, Lohmann J, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309:1056–1059
Samach A, Onouchi H, Gold S, Ditta G, Schwarz-Sommer Z, Yanofsky M, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288:1613–1616
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056
Mathieu J, Warthmann N, Küttner F, Schmid M (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17:1055–1060
Jäger K, Wigge P (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17:1050–1054
Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T (2008) Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol 49:1645–1658
Li C, Zhang K, Zeng X, Jackson S, Zhou Y, Hong Y (2009) A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J Virol 83:3540–3548
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43(10):1096–1105
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12(12):2473–2484
Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135:767–774
Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18(8):926–936. doi:10.1101/gad.1189604
Itoh H, Nonoue Y, Yano M, Izawa T (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42(7):635–638. doi:10.1038/ng.606
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316(5827):1033–1036. doi:10.1126/science.1141753
Hayama R, Agashe B, Luley E, King R, Coupland G (2007) A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis. Plant Cell 19:2988–3000
Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez J, Eshed Y (2006) The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA 103:6398–6403
Lifschitz E, Eshed Y (2006) Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. J Exp Bot 57:3405–3414
Alonso-Blanco C, Koornneef M (2000) Naturally occurring variation in Arabidopsis: an underexploited resource for plant genetics. Trends Plant Sci 5(1):22–29. S1360-1385(99)01510-1 [pii]
Napp-Zinn K (1987) Vernalization, environmental and genetic regulation. In: Atherton JG (ed) Manipulation of flowering. Butterworths, London, pp 123–132
Koornneef M, Blankestijn-de Vries H, Hanhart C, Soppe W, Peeters T (1994) The phenotype of some late-flowering mutants is enhanced by a locus on chromosome 5 that is not effective in the Landsberg erecta wild-type. Plant J 6:911–919
Lee I, Aukerman M, Gore S, Lohman K, Michaels S, Weaver L, John M, Feldmann K, Amasino R (1994) Isolation of LUMINIDEPENDENS: a gene involved in the control of flowering time in Arabidopsis. Plant Cell 6:75–83
Geraldo N, Bäurle I, Kidou S, Hu X, Dean C (2009) FRIGIDA delays flowering in Arabidopsis via a cotranscriptional mechanism involving direct interaction with the nuclear cap-binding complex. Plant Physiol 150:1611–1618
Searle I, He Y, Turck F, Vincent C, Fornara F, Krober S, Amasino RA, Coupland G (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20(7):898–912. doi:10.1101/gad.373506
Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES (1999) The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11(3):445–458
Helliwell C, Wood C, Robertson M, Peacock WJ, Dennis E (2006) The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J 46:183–192
Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G (2002) Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J 21(16):4327–4337
Michaels S, Amasino R (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13:935–941
Gendall A, Levy Y, Wilson A, Dean C (2001) The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107:525–535
Levy Y, Mesnage S, Mylne J, Gendall A, Dean C (2002) Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297:243–246
Sung S, Amasino R (2004) Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427:159–164
Bond D, Dennis E, Pogson B, Finnegan E (2009) Histone acetylation, VERNALIZATION INSENSITIVE 3, FLOWERING LOCUS C, and the vernalization response. Mol Plant 2:724–737
De Lucia F, Crevillen P, Jones AM, Greb T, Dean C (2008) A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci USA 105(44):16831–16836. doi:10.1073/pnas.0808687105
Wood CC, Robertson M, Tanner G, Peacock WJ, Dennis ES, Helliwell CA (2006) The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci USA 103(39):14631–14636. doi:10.1073/pnas.0606385103
Cao R, Zhang Y (2004) The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 14(2):155–164. doi:10.1016/j.gde.2004.02.001
Finnegan EJ, Dennis ES (2007) Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr Biol 17(22):1978–1983. doi:10.1016/j.cub.2007.10.026
Swiezewski S, Liu F, Magusin A, Dean C (2009) Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462:799–802
Heo JB, Sung S (2011) Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 331(6013):76–79. doi:10.1126/science.1197349
De Lucia F, Dean C (2010) Long non-coding RNAs and chromatin regulation. Curr Opin Plant Biol. doi:10.1016/j.pbi.2010.11.006
Simpson G (2004) The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opin Plant Biol 7:570–574
Liu F, Marquardt S, Lister C, Swiezewski S, Dean C (2010) Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327:94–97
Hecht V, Foucher F, Ferrandiz C, Macknight R, Navarro C, Morin J, Vardy ME, Ellis N, Beltran JP, Rameau C, Weller JL (2005) Conservation of Arabidopsis flowering genes in model legumes. Plant Physiol 137(4):1420–1434. doi:10.1104/pp.104.057018
Schläppi M, Patel M (2001) Biennialism and vernalization-promoted flowering in Hyoscyamus niger: a comparison with Arabidopsis. Flower Newsl 31:25–32
Tadege M, Sheldon CC, Helliwell CA, Upadhyaya NM, Dennis ES, Peacock WJ (2003) Reciprocal control of flowering time by OsSOC1 in transgenic Arabidopsis and by FLC in transgenic rice. Plant Biotechnol J 1(5):361–369. doi:10.1046/j.1467-7652.2003.00034.x
Wang R, Farrona S, Vincent C, Joecker A, Schoof H, Turck F, Alonso-Blanco C, Coupland G, Albani MC (2009) PEP1 regulates perennial flowering in Arabis alpina. Nature 459(7245):423–427. doi:10.1038/nature07988
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303(5664):1640–1644. doi:10.1126/science.1094305
Balasubramanian S, Sureshkumar S, Lempe J, Weigel D (2006) Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet 2:e106
Halliday KJ, Salter MG, Thingnaes E, Whitelam GC (2003) Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J 33(5):875–885
Blázquez MA, Ahn JH, Weigel D (2003) A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet 33(2):168–171. doi:10.1038/ng1085
Werner JD, Borevitz JO, Uhlenhaut NH, Ecker JR, Chory J, Weigel D (2005) FRIGIDA-independent variation in flowering time of natural Arabidopsis thaliana accessions. Genetics 170(3):1197–1207. doi:10.1534/genetics.104.036533
Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P (2000) Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J 21:351–360
Lee J, Yoo S, Park S, Hwang I, Lee J, Ahn J (2007) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev 21:397–402
Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell C, Ito T, Meyerowitz E, Yu H (2008) A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell 15:110–120
Kumar S, Wigge P (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140:136–147
Choi K, Kim S, Kim SY, Kim M, Hyun Y, Lee H, Choe S, Kim SG, Michaels S, Lee I (2005) SUPPRESSOR OF FRIGIDA3 encodes a nuclear ACTIN-RELATED PROTEIN6 required for floral repression in Arabidopsis. Plant Cell 17(10):2647–2660. doi:10.1105/tpc.105.035485
Deal RB, Kandasamy MK, McKinney EC, Meagher RB (2005) The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17(10):2633–2646. doi:10.1105/tpc.105.035196
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5(12):523–530
Koornneef M, van der Veen J (1980) Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet 58(6):257–263
Sun T, Goodman H, Ausubel F (1992) Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell 4:119–128
Koornneef M, Vaneden J, Hanhart CJ, Dejongh AMM (1983) Genetic fine-structure of the Ga-1 locus in the higher-plant Arabidopsis thaliana (L.) Heynh. Genet Res 41(1):57
Wilson R, Heckman J, Somerville C (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100:403–408
Jacobsen SE, Olszewski NE (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5(8):887–896. doi:10.1105/tpc.5.8.887
Shimada A, Ueguchi-Tanaka M, Sakamoto T, Fujioka S, Takatsuto S, Yoshida S, Sazuka T, Ashikari M, Matsuoka M (2006) The rice SPINDLY gene functions as a negative regulator of gibberellin signaling by controlling the suppressive function of the DELLA protein, SLR1, and modulating brassinosteroid synthesis. Plant J 48(3):390–402. doi:10.1111/j.1365-313X.2006.02875.x
Swain S, Tseng T, Olszewski N (2001) Altered expression of SPINDLY affects gibberellin response and plant development. Plant Physiol 126:1174–1185
Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T, Hsing Y, Kitano H, Yamaguchi I, Matsuoka M (2005) GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437:693–698
Griffiths J, Murase K, Rieu I, Zentella R, Zhang Z, Powers S, Gong F, Phillips A, Hedden P, Sun T, Thomas S (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18:3399–3414
Willige B, Ghosh S, Nill C, Zourelidou M, Dohmann E, Maier A, Schwechheimer C (2007) The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell 19:1209–1220
King RW, Moritz T, Evans LT, Junttila O, Herlt AJ (2001) Long-day induction of flowering in Lolium temulentum involves sequential increases in specific gibberellins at the shoot apex. Plant Physiol 127(2):624–632
Silverstone A, Ciampaglio C, Sun T (1998) The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell 10:155–169
Hisamatsu T, King R (2008) The nature of floral signals in Arabidopsis. II. Roles for FLOWERING LOCUS T (FT) and gibberellin. J Exp Bot 59:3821–3829
Harberd NP, Belfield E, Yasumura Y (2009) The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell 21(5):1328–1339. doi:10.1105/tpc.109.066969
Sun TP (2010) Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiol 154(2):567–570. doi:10.1104/pp.110.161554
Koornneef M, Elgersma A, Hanhart CJ, Vanloenenmartinet EP, Vanrijn L, Zeevaart JAD (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant 65(1):33–39
Murase K, Hirano Y, Sun T, Hakoshima T (2008) Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456:459–463
Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd N (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiol 143:1163–1172
Alvey L, Harberd N (2005) DELLA proteins: integrators of multiple plant growth regulatory inputs? Physiol Plant 123(2):153–160. doi:10.1111/j.1399-3054.2004.00412.x
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421(6924):740–743. doi:10.1038/nature01387
de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451(7177):480–484. doi:10.1038/nature06520
Schwechheimer C, Willige BC (2009) Shedding light on gibberellic acid signalling. Curr Opin Plant Biol 12(1):57–62. doi:10.1016/j.pbi.2008.09.004
Oda A, Fujiwara S, Kamada H, Coupland G, Mizoguchi T (2004) Antisense suppression of the Arabidopsis PIF3 gene does not affect circadian rhythms but causes early flowering and increases FT expression. FEBS Lett 557(1–3):259–264. S0014579303014704 [pii]
Blázquez MA, Soowal LN, Lee I, Weigel D (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124(19):3835–3844
Blázquez M, Green R, Nilsson O, Sussman M, Weigel D (1998) Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10:791–800
Eriksson S, Böhlenius H, Moritz T, Nilsson O (2006) GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 18:2172–2181
Blázquez M, Weigel D (2000) Integration of floral inductive signals in Arabidopsis. Nature 404:889–892
Gocal G, Sheldon C, Gubler F, Moritz T, Bagnall D, MacMillan C, Li S, Parish R, Dennis E, Weigel D, King R (2001) GAMYB-like genes, flowering, and gibberellin signaling in Arabidopsis. Plant Physiol 127:1682–1693
Gocal G, Poole A, Gubler F, Watts R, Blundell C, King R (1999) Long-day up-regulation of a GAMYB gene during Lolium temulentum inflorescence formation. Plant Physiol 119:1271–1278
Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425(6955):257–263. doi:10.1038/nature01958
Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12(17):1484–1495. S0960982202010175 [pii]
Achard P, Herr A, Baulcombe D, Harberd N (2004) Modulation of floral development by a gibberellin-regulated microRNA. Development 131:3357–3365
Moon J, Suh S, Lee H, Choi K, Hong C, Paek N, Kim S, Lee I (2003) The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J 35:613–623
Achard P, Baghour M, Chapple A, Hedden P, Van Der Straeten D, Genschik P, Moritz T, Harberd N (2007) The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proc Natl Acad Sci USA 104:6484–6489
Yu H, Xu Y, Tan E, Kumar P (2002) AGAMOUS-LIKE 24, a dosage-dependent mediator of the flowering signals. Proc Natl Acad Sci USA 99:16336–16341
Liu C, Chen H, Er H, Soo H, Kumar P, Han J, Liou Y, Yu H (2008) Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135:1481–1491
Richter R, Behringer C, Müller I, Schwechheimer C (2010) The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 24:2093–2104
Madhusudanan KN, Nandakumar S (1983) Carbohydrate changes in shoot tip and subtending leaves during ontogenetic development of pineapple. Z Pflanzenphysiol 110(5):429–438
Komarova EN, Milyaeva EL (1991) Changes in content and distribution of starch in stem apices of bicolored coneflower during the period of flowering evocation. Sov Plant Physiol 38(1):46–51
Lejeune P, Bernier G, Requier MC, Kinet JM (1993) Sucrose increase during floral induction in the phloem sap collected at the apical part of the shoot of the long-day plant Sinapis alba L. Planta 190(1):71–74
King RW, Evans LT (1991) Shoot apex sugars in relation to long-day induction of flowering in Lolium temulentum L. Aust J Plant Physiol 18(2):121–135
Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K (2001) Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol 127:252–261
Roldán M, Gómez-Mena C, Ruiz-García L, Salinas J, Martínez-Zapater J (1999) Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J 20:581–590
El-Lithy M, Reymond M, Stich B, Koornneef M, Vreugdenhil D (2010) Relation among plant growth, carbohydrates and flowering time in the Arabidopsis Landsberg erecta × Kondara recombinant inbred line population. Plant Cell Environ 33(8):1369–1382
Caspar T, Huber SC, Somerville C (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79(1):11–17
Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C (1991) Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiol 95(4):1181–1188
Corbesier L, Lejeune P, Bernier G (1998) The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta 206:131–137
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441. doi:10.1146/annurev.arplant.59.032607.092945
Eastmond PJ, van Dijken AJ, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JD, Smeekens SC, Graham IA (2002) Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J 29(2):225–235. 1220 [pii]
van Dijken AJ, Schluepmann H, Smeekens SC (2004) Arabidopsis trehalose-6-phosphate synthase 1 is essential for normal vegetative growth and transition to flowering. Plant Physiol 135(2):969–977. doi:10.1104/pp.104.039743
Wang J, Czech B, Weigel D (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138:738–749
Noh B, Lee SH, Kim HJ, Yi G, Shin EA, Lee M, Jung KJ, Doyle MR, Amasino RM, Noh YS (2004) Divergent roles of a pair of homologous jumonji/zinc-finger-class transcription factor proteins in the regulation of Arabidopsis flowering time. Plant Cell 16(10):2601–2613. doi:10.1105/tpc.104.025353
Koornneef M, Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Peeters AJ (1998) Genetic interactions among late-flowering mutants of Arabidopsis. Genetics 148(2):885–892
He Y, Michaels S, Amasino R (2003) Regulation of flowering time by histone acetylation in Arabidopsis. Science 302:1751–1754
Liu F, Quesada V, Crevillén P, Bäurle I, Swiezewski S, Dean C (2007) The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol Cell 28:398–407
Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbett C, Dean C (1997) FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89:737–745
Quesada V, Macknight R, Dean C, Simpson G (2003) Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time. EMBO J 22:3142–3152
Ausín I, Alonso-Blanco C, Jarillo J, Ruiz-García L, Martínez-Zapater J (2004) Regulation of flowering time by FVE, a retinoblastoma-associated protein. Nat Genet 36:162–166
Schomburg F, Patton D, Meinke D, Amasino R (2001) FPA, a gene involved in floral induction in Arabidopsis, encodes a protein containing RNA-recognition motifs. Plant Cell 13:1427–1436
Bäurle I, Smith L, Baulcombe D, Dean C (2007) Widespread role for the flowering-time regulators FCA and FPA in RNA-mediated chromatin silencing. Science 318:109–112
Hornyik C, Terzi LC, Simpson GG (2010) The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell 18(2):203–213. doi:10.1016/j.devcel.2009.12.009
Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C (2003) FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 113(6):777–787. S0092867403004252 [pii]
Adams S, Allen T, Whitelam GC (2009) Interaction between the light quality and flowering time pathways in Arabidopsis. Plant J 60(2):257–267. doi:10.1111/j.1365-313X.2009.03962.x
Lim MH, Kim J, Kim YS, Chung KS, Seo YH, Lee I, Hong CB, Kim HJ, Park CM (2004) A new Arabidopsis gene, FLK, encodes an RNA binding protein with K homology motifs and regulates flowering time via FLOWERING LOCUS C. Plant Cell 16(3):731–740. doi:10.1105/tpc.019331
Aukerman M, Lee I, Weigel D, Amasino R (1999) The Arabidopsis flowering-time gene LUMINIDEPENDENS is expressed primarily in regions of cell proliferation and encodes a nuclear protein that regulates LEAFY expression. Plant J 18:195–203
Kim S, Choi K, Park C, Hwang HJ, Lee I (2006) SUPPRESSOR OF FRIGIDA4, encoding a C2H2-type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis FLOWERING LOCUS C. Plant Cell 18(11):2985–2998. doi:10.1105/tpc.106.045179
Simpson G, Dean C (2002) Arabidopsis, the Rosetta stone of flowering time? Science 296:285–289
Kim S, Soltis PS, Wall K, Soltis DE (2006) Phylogeny and domain evolution in the APETALA2-like gene family. Mol Biol Evol 23(1):107–120. doi:10.1093/molbev/msj014
Mathieu J, Yant LJ, Murdter F, Küttner F, Schmid M (2009) Repression of flowering by the miR172 target SMZ. PLoS Biol 7(7):e1000148. doi:10.1371/journal.pbio.1000148
Yant L, Mathieu J, Dinh T, Ott F, Lanz C, Wollmann H, Chen X, Schmid M (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22:2156–2170
Castillejo C, Pelaz S (2008) The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr Biol 18(17):1338–1343. doi:10.1016/j.cub.2008.07.075
Lee J, Oh M, Park H, Lee I (2008) SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J 55(5):832–843. doi:10.1111/j.1365-313X.2008.03552.x
Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM (1992) LEAFY controls floral meristem identity in Arabidopsis. Cell 69(5):843–859
Weigel D, Nilsson O (1995) A developmental switch sufficient for flower initiation in diverse plants. Nature 377(6549):495–500. doi:10.1038/377495a0
Yu H, Ito T, Wellmer F, Meyerowitz E (2004) Repression of AGAMOUS-LIKE 24 is a crucial step in promoting flower development. Nat Genet 36:157–161
Liu C, Xi W, Shen L, Tan C, Yu H (2009) Regulation of floral patterning by flowering time genes. Dev Cell 16(5):711–722. doi:10.1016/j.devcel.2009.03.011
Wagner D (2009) Flower morphogenesis: timing is key. Dev Cell 16(5):621–622. doi:10.1016/j.devcel.2009.05.005
Liu Z, Mara C (2010) Regulatory mechanisms for floral homeotic gene expression. Semin Cell Dev Biol 21:80–86
Moyroud E, Kusters E, Monniaux M, Koes R, Parcy F (2010) LEAFY blossoms. Trends Plant Sci 15(6):346–352. doi:10.1016/j.tplants.2010.03.007
Gregis V, Sessa A, Colombo L, Kater MM (2008) AGAMOUS-LIKE24 and SHORT VEGETATIVE PHASE determine floral meristem identity in Arabidopsis. Plant J 56(6):891–902. doi:10.1111/j.1365-313X.2008.03648.x
Kaufmann K, Wellmer F, Muiño J, Ferrier T, Wuest S, Kumar V, Serrano-Mislata A, Madueño F, Krajewski P, Meyerowitz E, Angenent G, Riechmann J (2010) Orchestration of floral initiation by APETALA1. Science 328:85–89
Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM (2003) AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J 33(5):867–874
Yant L, Mathieu J, Schmid M (2009) Just say no: floral repressors help Arabidopsis bide the time. Curr Opin Plant Biol 12(5):580–586. doi:10.1016/j.pbi.2009.07.006
Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B (2000) FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101(3):331–340
Lee I, Michaels SD, Masshardt AS RMA (1994) The late-flowering phenotype of FRIGIDA and LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J 6:903–909
Scortecci KC, Michaels SD, Amasino RM (2001) Identification of a MADS-box gene, FLOWERING LOCUS M, that represses flowering. Plant J 26(2):229–236
Kim HJ, Hyun Y, Park JY, Park MJ, Park MK, Kim MD, Lee MH, Moon J, Lee I, Kim J (2004) A genetic link between cold responses and flowering time through FVE in Arabidopsis thaliana. Nat Genet 36(2):167–171. doi:10.1038/ng1298
Henderson IR, Liu F, Drea S, Simpson GG, Dean C (2005) An allelic series reveals essential roles for FY in plant development in addition to flowering-time control. Development 132(16):3597–3607. doi:10.1242/dev.01924
Koornneef M, Rolff E, Spruit CJP (1980) Genetic-control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol 100(2):147–160
Rubio V, Deng XW (2007) Plant science. Standing on the shoulders of GIGANTEA. Science 318(5848):206–207. doi:10.1126/science.1150213
Gaudin V, Libault M, Pouteau S, Juul T, Zhao G, Lefebvre D, Grandjean O (2001) Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 128(23):4847–4858
Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61:2247–2254
Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138(4):750–759. doi:10.1016/j.cell.2009.06.031
Yamaguchi A, Wu MF, Yang L, Wu G, Poethig RS, Wagner D (2009) The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell 17(2):268–278. doi:10.1016/j.devcel.2009.06.007
Tseng T, Swain S, Olszewski N (2001) Ectopic expression of the tetratricopeptide repeat domain of SPINDLY causes defects in gibberellin response. Plant Physiol 126:1250–1258
Blázquez M, Santos E, Flores C, Martínez-Zapater J, Salinas J, Gancedo C (1998) Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J 13:685–689
Vogel G, Aeschbacher R, Müller J, Boller T, Wiemken A (1998) Trehalose-6-phosphate phosphatases from Arabidopsis thaliana: identification by functional complementation of the yeast tps2 mutant. Plant J 13:673–683
Acknowledgments
The authors would like to thank Dr. Yasushi Kobayashi, Dr. Sureshkumar Balasubramanian, Dr. Jia Wei Wang, Dr. Lisa Smith, Dr. Dan Koenig, Dr. Beth Rowan, Dr. George Wang and Vinicius Galvao for comments on the manuscript and the Max-Planck Gesellschaft for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srikanth, A., Schmid, M. Regulation of flowering time: all roads lead to Rome. Cell. Mol. Life Sci. 68, 2013–2037 (2011). https://doi.org/10.1007/s00018-011-0673-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-011-0673-y