Abstract.
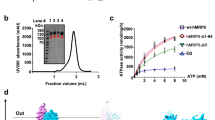
Human ABCG2 was efficiently overexpressed in insect cell membranes, solubilized with 3-[(3-cholamidopropyl)dimethyl ammonio]-1-propanesulfonate, and purified through N-terminal hexahistidine tag. Its functionality was assessed by high vanadate-sensitive ATPase activity, and nucleotide-binding capacity. Interestingly, the R482T point mutation increased both maximal hydrolysis rate and affinity for MgATP, and lowered sensitivity to vanadate inhibition. Direct nucleotide binding, as monitored by quenching of intrinsic fluorescence, indicated a mutation-related preference for ATP over ADP. The R482T mutation only produced a limited change, if any, on the binding of drug substrates, indicating that methotrexate, on the one hand, and rhodamine 123 or doxorubicin, on the other hand, bound similarly to wild-type and mutant transporters whether or not they were subject to cellular transport. In addition, the characteristic inhibitors GF120918 and 6-prenylchrysin, which alter mitoxantrone efflux much better for wild-type than mutant ABCG2, bound similarly to purified ABCG2, while the highly-potent Ko143 bound in the nanomolar range also effective in inhibition of drug transport. All results indicate that the role of the arginine-482 mutation on substrate drug transport and inhibitor efficiency is not mediated by changes in drug binding.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Additional information
Received 10 April 2006; received after revision 22 May 2006; accepted 12 June 2006
A. Pozza and J. M. Perez-Victoria contributed equally to this work
Rights and permissions
About this article
Cite this article
Pozza, A., Perez-Victoria, J.M., Sardo, A. et al. Purification of breast cancer resistance protein ABCG2 and role of arginine-482. Cell. Mol. Life Sci. 63, 1912–1922 (2006). https://doi.org/10.1007/s00018-006-6159-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-006-6159-7