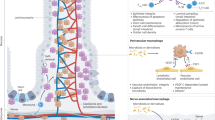
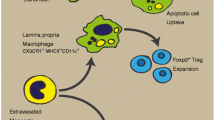
Abstract
Gut homeostasis is a process that requires a prudent balance of host responses to the beneficial enteric microbial community and the pathogenic stimuli that can arise. The lack of this balance in the intestine can result in inflammatory bowel diseases, where the immune system dysfunctions leading to exacerbated inflammatory responses. In this process, macrophages are considered to play a pivotal role. In this review, we describe the important role of macrophages in maintaining intestinal homeostasis and we discuss how altered macrophage function may lead to inflammatory bowel diseases. The plasticity of macrophages during the gut inflammatory response shows the broad role of these cells in orchestrating not only the onset of inflammation but also its termination as well as healing and repair. Indeed, the state of macrophage polarization can be the key factor in defining the resolution or the progression of inflammation and disease. Here, we discuss the different populations of macrophages and their implication in development, propagation, control and resolution of inflammatory bowel diseases.


Similar content being viewed by others
References
Gordon S, Crocker PR, Morris L, Lee SH, Perry VH, Hume DA. Localization and function of tissue macrophages. Biochem macrophages. Chichester: John Wiley & Sons Ltd.; 1986. p. 54–67.
Matzinger P. Tolerance, danger, and the extended family. Annu Rev lmmunol. 1994;12:991–1045.
Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13.
Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Elem Immun. 1996;272:50–4.
Silverstein SC. Phagocytosis of microbes: insights and prospects. Trends Cell Biol. 1995;5:141–2.
Allen L-AH, Aderem A. Mechanisms of phagocytosis. Curr Opin Immunol. 1996;8:36–40.
Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–78.
Metschnikoff E. Ueber den Kampf der Zellen gegen Erysipel-kokken-Ein Beitrag zur Phagocytenlehre. Arch für Pathol Anat Physiol für Klin Med. 1887;107:209–49.
Tauber AI. Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Biol. 2003;4:897–901.
Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015. https://doi.org/10.1146/annurev-immunol-032414-112220.
Platt AM, Mowat AMI. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett. 2008;119:22–31.
Grainger JR, Konkel JE, Zangerle-Murray T, Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch Eur J Physiol. 2017;469:527–39.
Gren ST, Grip O. Role of monocytes and intestinal macrophages in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:1992–8.
Lissner D, Schumann M, Batra A, Kredel L-I, Kühl AA, Erben U, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297–305.
Podolsky DK. Inflammatory Bowel disease. N Engl J Med. 1991;325:928–37. https://doi.org/10.1056/NEJM199309303291401.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34.
Graff LA, Walker JR, Bernstein CN. Depression and anxiety in iflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15:1105–18.
Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65.
Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–53.
Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci USA. 2005;102:99–104.
Yan F, Wang L, Shi Y, Cao H, Liu L, Washington MK, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. AJP Gastrointest Liver Physiol. 2012;302:G504–G514514. https://doi.org/10.1152/ajpgi.00312.2011.
Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362–81.
Seo DH, Che X, Kwak MS, Kim S, Kim JH, Ma HW, et al. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-00840-2.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–41. https://doi.org/10.1016/j.cell.2014.03.011.
Nagashima R, Maeda K, Imai Y, Takahashi T. Lamina propria macrophages in the human gastrointestinal mucosa: their distribution, immunohistological phenotype, and function. J Histochem Cytochem. 1996;44:721–31.
Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut. 1998;42:470–6.
Powell N, Walker AW, Stolarczyk E, Canavan JB, Gökmen MR, Marks E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid Cells. Immunity. 2012;37:674–84.
Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol. 2008;1:339–49.
Gerlach K, Hwang Y, Nikolaev A, Atreya R, Dornhoff H, Steiner S, et al. T H 9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676–86.
Schmidt A, Oberle N, Krammer PH. Molecular mechanisms oftreg-mediatedt cell suppression. Front Immunol. 2012. https://doi.org/10.3389/fimmu.2012.00051.
Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50.
Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711–22. https://doi.org/10.1016/j.chom.2013.05.013.
Anderson P, Souza-Moreira L, Morell M, Caro M, O’Valle F, Gonzalez-Rey E, et al. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013;62:1131–41.
Kozicky LK, Menzies SC, Hotte N, Madsen KL, Sly LM. Intravenous immunoglobulin (IVIg) or IVIg-treated macrophages reduce DSS-induced colitis by inducing macrophage IL-10 production. Eur J Immunol. 2019. https://doi.org/10.1002/eji.201848014.
Tiemessen MM, Jagger AL, Evans HG, Van Herwijnen MJC, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci. 2007;104:19446–51.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Mackaness GB. Cellular Immunity and the parasite. Adv Exp Med Biol. 1977;93:65–73.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:1–13.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. https://doi.org/10.1016/j.immuni.2014.06.008.
Murray HW, Spitalny GL, Nathan CF. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985;134:1619–22.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35.
Stein M, SK, Harris N, Gordon S, Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–92.
Sutterwala BFS, Noel GJ, Salgame P, Mosser DM. Macrophage Fcg receptor Type I. J Exp Med. 1998;188:217–22.
Fleming BD, Mosser DM. Regulatory macrophages: setting the threshold for therapy. Eur J Immunol. 2011;41:2498–502.
Gerber JS, Mosser DM. Reversing lipopolysaccharide toxicity by ligating the macrophage Fcγ receptors. J Immunol. 2001;166:6861–8.
Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12.
Mosser DM, Gonçalves R. Activation of murine macrophages. Curr Protoc Immunol. 2015. https://doi.org/10.1002/0471142735.im1402s111.
Bull DM, Bookman MA. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966–74.
Hume DA, Loutit JF, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984;66:189–94.
Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475–89.
Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–37.
De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(400–415):e13.
Shaw TN, Houston SA, Wemyss K, Bridgeman HM, Barbera TA, Zangerle-Murray T, et al. Tissue-resident macrophages in the intestine are long lived and defined by Tim-4 and CD4 expression. J Exp Med. 2018;215:1507–18.
Bain CC, Mowat AMI. Macrophages in gastrointestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–17.
Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6C hi monocyte precursors. Mucosal Immunol. 2013;6:498–510.
Hirotani T, Lee PY, Kuwata H, Yamamoto M, Matsumoto M, Kawase I, et al. The nuclear IκB protein IκBNS selectively inhibits lipopolysaccharide-induced IL-6 production in macrophages of the colonic lamina propria. J Immunol. 2005;174:3650–7.
Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–84.
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–85.
Gordon S, Pluddemann A, Martinez EF. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262:36–55.
Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–74.
Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40–50.
Grimm M, Pullman W, Bennett G, Sullivan P, Pavli P, Doe W. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995;10:387–95.
Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184:6843–54.
Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33.
Sperber K, Ogata S, Sylvester C, Aisenberg J, Chen A, Mayer L, et al. A novel human macrophage-derived intestinal mucin secretagogue: implications for the pathogenesis of inflammatory bowel disease. Gastroenterology. 1993;104:1302–9.
Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–90. https://doi.org/10.1016/j.immuni.2012.08.026.
Schwarzmaier D, Foell D, Weinhage T, Varga G, Däbritz J. Peripheral monocyte functions and activation in patients with quiescent Crohn’s disease. PLoS ONE. 2013;8:8–14.
Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis. 2005;11:580–8.
Sanchez-Muñoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–8.
Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306.
Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–41.
Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–19.
Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–33.
Eddie Ip WK, Hoshi N, Shouval DD, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science (80-). 2017;356:513–9.
Mantovani A, Marchesi F. IL-10 and macrophages orchestrate gut homeostasis. Immunity. 2014;40:637–9. https://doi.org/10.1016/j.immuni.2014.04.015.
Keubler LM, Buettner M, Häger C, Bleich A. A multihit model: colitis lessons from the interleukin-10-deficient mouse. Inflamm Bowel Dis. 2015;21:1967–75.
Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74.
Li B, Alli R, Vogel P, Geiger TL. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014;7:869–78.
Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. Gastroenterology. 2000;119:1473–82.
Lindsay JO, Hodgson HJF. Review article: the immunoregulatory cytokine interleukin-10. A therapy for Crohn’s disease? Aliment Pharmacol Ther. 2001;15:1709–16.
Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434–44.
Marlow GJ, van Gent D, Ferguson LR. Why interleukin-10 supplementation does not work in Crohn’s disease patients. World J Gastroenterol. 2013;19:3931–41.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Fleming BD, Chandrasekaran P, Dillon LAL, Dalby E, Suresh R, Sarkar A, et al. The generation of macrophages with anti-inflammatory activity in the absence of STAT6 signaling. J Leukoc Biol. 2015;98:395–407.
Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–15.
Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie ANJ, Van Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–666.
Chang H-H, Miaw S-C, Tseng W, Sun Y-W, Liu C-C, Tsao H-W, et al. PTPN22 modulates macrophage polarization and susceptibility to dextran sulfate sodium-induced colitis. J Immunol. 2013;191:2134–43. https://doi.org/10.4049/jimmunol.1203363.
Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis. 2014;20:166–75.
Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, et al. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J Clin Invest. 2008;118:2269–80.
Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin Exp Immunol. 1993;94:174–81.
Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012. https://doi.org/10.1084/jem.20101387.
Hunter MM, Wang A, Parhar KS, Johnston MJG, Van Rooijen N, Beck PL, et al. in vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–405. https://doi.org/10.1053/j.gastro.2009.12.041.
Leung G, Wang A, Fernando M, Phan VC, McKay DM. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: participation of IL-10. Am J Physiol Gastrointest Liver Physiol. 2013;304:G781–G792792.
Weisser SB, Brugger HK, Voglmaier NS, McLarren KW, van Rooijen N, Sly LM. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J Leukoc Biol. 2011;90:483–92.
Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. 2015;6:613.
Chandrasekaran P, Izadjoo S, Stimely J, Palaniyandi S, Zhu X, Tafuri W, et al. Regulatory macrophages inhibit alternative macrophage activation and attenuate pathology associated with fibrosis. J Immunol. 2019;203:2130–40.
Fernández-Hernando C, Ackah E, Yu J, Suárez Y, Murata T, Iwakiri Y, et al. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–57.
Franke TF, Yang S, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–36.
Arranz A, Doxaki C, Vergadi E, de la Martinez YT, Vaporidi K, Lagoudaki ED, et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci. 2012;109:517–22.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Grant no. [RED-00570-16] and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Grant no. [Scholarship].
Author information
Authors and Affiliations
Contributions
TL and RG wrote the article and create the figures. DM wrote the article.
Corresponding author
Additional information
Responsible Editor: H. Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreira Lopes, T.C., Mosser, D.M. & Gonçalves, R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 69, 1163–1172 (2020). https://doi.org/10.1007/s00011-020-01398-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01398-y