Abstract
Background
Intermittent hypoxia (IH), a hallmark of obstructive sleep apnea (OSA), is prevalent in older adults and associated with inflammation. We previously showed that IH induces renal fibrosis and cardiomyopathy and hypothesized that lung inflammatory changes may underlie deficits in pulmonary function in OSA.
Methods
Pulmonary inflammatory and oxidative markers were assessed in metallothionein KO (MT-KO) mice and WT 129S1 controls exposed to IH or to normoxia for 8 weeks.
Results
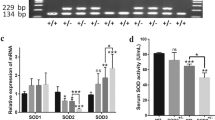
MT expression increased at 3 days in WT, falling back at 1 week. Pro-fibrotic markers CTGF and PAI-1 were unchanged in WT, but increased at 3 or 8 weeks, with enhanced Sirius Red staining at 8 weeks, in IH-exposed MT-KO. Cellular infiltration, TNF-α and IL-6 increased earlier in IH-exposed MT-KO than in WT. Oxidative markers, 3-nitrotyrosine and 4-hydroxynonenal increased in both but persisted in MT-KO. Antioxidant Nrf2, HO-1 and NQO1, increased at 3 days in WT mice and at 8 weeks IH in MT-KO. While early Nrf2 induction required MT, its later increase at 8 weeks in MT-KO was independent from MT.
Conclusions
We conclude that early MT and antioxidant gene response protects from fibrotic changes in long-term IH-exposed mouse lung. Without this response, pulmonary fibrosis may develop with longer IH exposure.







Similar content being viewed by others
References
Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:237–41.
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43.
Yin X, Zheng Y, Liu Q, Cai J, Cai L. Cardiac response to chronic intermittent hypoxia with a transition from adaptation to maladaptation: the role of hydrogen peroxide. Oxid Med Cell Longev. 2012;2012:569520.
Peppard PE, Young T, Barnet JH, Palta M, Hagen EM, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14.
Garvey JF, Perego MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7(5):920–9.
Ingram DG, Singh AV, Ehsan Z, Birnbaum BF. Obstructive sleep Apnea and pulmonary hypertensionin children. Paediatr Respir Rev. 2017;23:33–9.
Khayat R, Patt B, Hayes D Jr. Obstructive sleep apnea: the new cardiovascular disease. Part I: obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev. 2009;14(3):143–53.
Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132(1):325–37.
Dematteis M, Julien C, Guillermet C, Sturm N, Latuejoul S, Mallaret M, Levy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177:227–35.
Dematteis M, Godin-Ribuot D, Arnaud C, Ribuot C, Stanke-Labesque F, Pepin JL, Levy P. Cardiovascular consequences of sleep-disordered breathing: contribution of animal models to understanding the human disease. ILAR J. 2009;50(3):262–81.
Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, Lavie L, Pépin JL. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:1–20.
Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia—Revisited—The bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015;20:27–45.
Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad Med J. 2009;85(1010):693–8.
Aihara K, Oga T, Harada Y, Chihara Y, Handa T, Tanizawa K, Watanabe K, Tsuboi T, Mishima M, Chin K. Comparison of biomarkers of subclinical lung injury in obstructive sleep apnea. Resp Med. 2011;105:939–45.
da Rosa PD, Forgiarini LF, Baronio D, Feijo CA, Martinez D, Marroni NP. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm. 2012;2012:879419.
Schiza S, Mermigkis C, Margaritopoulos GA, Daniil Z, Harari S, Poletti V, Renzoni EA, Torre O, Visca D, Bouloukaki I, Sourvinos G, Antoniou KM. Idiopathic pulmonary fibrosis and sleep disorders: no longer strangers in the night. Eur Respir Rev. 2015;24(136):327–39.
Lu W, Kang K, Hu S, Tang X, Zhou S, Yu Y, Li Y, Xu L. Angiotensin-(1-7) inhibits inflammation and oxidative stress to relieve lung injury induced by chronic intermittent hypoxia in rats. Braz J Med Biol Res. 2016;49(10):e5431.
Kim JS, Podolanczuk AJ, Borker P, Kawu SM, Raghu G, Kaufman JD, Hinckley Stukovsky KD, Hoffman EA, Barr RG, Gottlieb DJ, Redline S, Lederer DJ. Obstructive sleep apnea and subclinical interstitial lung Disease in MESA. Ann Am Thorac Soc. 2017. https://doi.org/10.1513/annalsats.201701-091oc.
Beguin PC, Belaidi E, Godin-Ribuot D, Levy P, Ribuot C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 MAP kinase and Erk1/2. J Mol Cell Cardiol. 2007;42(2):343–51.
Gao C, Wang C, Liu B, Wu H, Yang Q, Jin J, Li H, Dong Gao SG, Zhang H. Intermittent hypoxia preconditioning-induced epileptic tolerance by upregulation of monocarboxylate transporter 4 expression in rat hippocampal astrocytes. Neurochem Res. 2014;39(11):2160–9.
Xu XM, Yao D, Cai XD, Ding C, Lin QD, Wang LX, Huang XY. Effect of chronic continual- and intermittent hypoxia-induced systemic inflammation on the cardiovascular system in rats. Sleep Breath. 2015;19:677–84.
Williams A, Scharf SM. Obstructive sleep apnea, cardiovascular disease, and inflammation—is NF-kB the key? Sleep Breath. 2007;11(2):69–76.
Htoo AK, Greenberg H, Tongia S, Chen G, Henderson T, Wilson D, Liu SF. Activation of nuclear factor kB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10(1):43–50.
Sun W, Yin X, Wang Y, Tan Y, Cai L, Wang B, Cai J, Fu Y. Intermittent hypoxia-induced renal antioxidants and oxidative damage in male mice: hermetic dose response. Dose Res. 2013;11:385–400.
Gozal E, Gozal D. Respiratory plasticity following intermittent hypoxia: developmental interactions. J Appl Physiol. 2001;90:1995–9.
Rosamond W, Hong Y. Heart disease and stroke statistics—2007 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115(5):69–171.
Lederer DJ, Jelic S, Basner RC, Ishizaka A, Bhattacharya J. Circulating KL-6, a biomarker of lung injury, in obstructive sleep apnea. Eur Respir J. 2009;33(4):793–6.
Tuleta I, Stockigt F, Juergens UR, Pizarro C, Schrickel JW, Kristiansen G, Nickenig G, Skowasch D. Intermittent hypoxia contributes to the lung damage by increased oxidative stress, inflammation, and disbalance in protease/antiprotease system. Lung. 2016;194:1015–20.
Kimura T, Kambe T. The functions of metallothionein and ZIP and ZNT transporters: an overview and perspective. Int J Mol Sci. 2016;17(3):336.
Cai Satoh LM, Tohyama C, Cherian MG. Metallothionein in radiation exposure: its induction and protective role. Toxicology. 1999;132(2–3):85–98.
Cai L. Diabetic cardiomyopathy and its prevention by metallothionein: experimental evidence, possible mechanisms and clinical implications. Curr Med Chem. 2007;14(20):2193–203.
Kang YJ. The antioxidant function of metallothionein in the heart. Proc Soc Exp Biol Med. 1999;222(3):263–73.
Yin X, Zhou S, Zheng Y, Tan Y, Kong M, Wang B, Feng W, Epstein PN, Cai J, Cai L. Metallothionein as a compensatory component prevents intermittent hypoxia-induced cardiomyopathy in mice. Toxicol Appl Pharmacol. 2014;277(1):58–66.
Zhou G, Li X, Hein DW, Xiang X, Marshall JP, Prabhu SD, Cai L. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–66.
Wu H, Zhou S, Kong L, Chen J, Feng W, Cai J, Miao L, Tan Y. Metallothionein deletion exacerbates intermittent hypoxia-induced renal injury in mice. Tox Lett. 2015;232:340–8.
Wu H, Kong L, Cheng Y, Zhang Z, Wang Y, Luo M, Tan Y, Chen X, Miao L, Cai L. Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2. Free Rad Biol Med. 2015;89:431–42.
Kojimka I, Tanaka T, Inagi R, Nishi H, Aburatani H, Kato H, Myata T, Fujita T, Nangaku M. Metallothionein is upregulated by hypoxia and stabilizes hypoxia-inducible factor in the kidney. Kidney Int. 2009;75:268–77.
Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Millstone AP, Collard HR, Mallow BA. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136(3):772–8.
Zhou S, Yin X, Jin J, Tan Y, Conklin DJ, Xin Y, Zhang Z, Sun W, Cui T, Cai J, Zheng Y, Cai L. Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein. Free Rad Biol Med. 2017;112:224–39.
Gozal E, Gozal D, Pierce WM, Thongboonkerd V, Scherzer JA, Sachleben LR Jr, Guo SZ, Cai J, Klein JB. Proteomic analysis of CA1 and CA3 regions of rat hippocampus and differential susceptibility to intermittent hypoxia. J Neurochem. 2002;83:1–14.
Cai J, Tuong CM, Gozal D. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir Physiol Neurobiol. 2011;178(2):210–7.
Cai J, Tuong CM, Zhang Y, Shields CB, Guo G, Fu H, Gozal D. Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. J Pathol. 2012;226(3):495–508.
Yang J, Tan Y, Zhao F, Ma Z, Wang Y, Zheng S, Epstein PN, Yu J, Yin X, Zheng Y, Li X, Miao L, Cai L. Angiotensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of NADPH oxidase-mediated nitrosative damage. Am J Physiol Endocrinol Metab. 2011;301:E132–44.
Braun RK, Broytman O, Braun FM, Brinkman JA, Clithero A, Pegelow DF, Eldridge M, Teodorescu M. Chronic intermittent hypoxia worsens bleomycin-induced lung fibrosis in rats. Resp Physiol Neurobiol. 2017;256:97–108.
Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL, Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. Am J Respir Cell Mol Biol. 2009;40:601–9.
Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113(4):544–54.
Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54(6):1829–37.
Funding
This study was supported in part by a University of Louisville Collaborative Matching Grant (to EG), 001GN09-Sleep Research Society Foundation/J. Christian Gillin M.D. (to JC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, X., Jagadapillai, R., Cai, J. et al. Metallothionein induction attenuates the progression of lung injury in mice exposed to long-term intermittent hypoxia. Inflamm. Res. 69, 15–26 (2020). https://doi.org/10.1007/s00011-019-01287-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-019-01287-z