Abstract
Objective
To evaluate the effect and mechanisms of naringenin in TiO2-induced chronic arthritis in mice, a model resembling prosthesis and implant inflammation.
Treatment
Flavonoids are antioxidant and anti-inflammatory molecules with important anti-inflammatory effect. Mice were daily treated with the flavonoid naringenin (16.7–150 mg/kg, orally) for 30 days starting 24 h after intra-articular knee injection of 3 mg of TiO2.
Methods
TiO2-induced arthritis resembles cases of aseptic inflammation induced by prosthesis and/or implants. Mice were stimulated with 3 mg of TiO2 and after 24 h mice started to be treated with naringenin. The disease phenotype, treatment toxicity, histopathological damage, oxidative stress, cytokine expression and NFκB were evaluated after 30 days of treatment.
Results
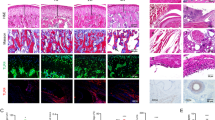
Naringenin inhibited TiO2-induced mechanical hyperalgesia (96%), edema (77%) and leukocyte recruitment (74%) without inducing toxicity. Naringenin inhibited histopathological index (HE, 49%), cartilage damage (Toluidine blue tibial staining 49%, and proteoglycan 98%), and bone resorption (TRAP-stained 73%). These effects were accompanied by inhibition of oxidative stress (gp91phox 93%, NBT 83%, and TBARS 41%) cytokine mRNA expression (IL-33 82%, TNFα 76%, pro-IL-1β 100%, and IL-6 61%), and NFκB activation (100%).
Conclusion
Naringenin ameliorates TiO2-induced chronic arthritis inducing analgesic and anti-inflammatory responses with improvement in the histopathological index, cartilage damage, and bone resorption.








Similar content being viewed by others
References
Siddiqui MMA, Yeo SJ, Sivaiah P, Chia S-L, Chin PL, Lo NN. Function and quality of life in patients with recurvatum deformity after primary total knee arthroplasty: a review of our joint registry. J Arthroplasty. 2012;27(6):1106–10. https://doi.org/10.1016/j.arth.2011.10.013.
Soever LJ, Mackay C, Saryeddine T, Davis AM, Flannery JF, Jaglal SB, et al. Educational needs of patients undergoing total joint arthroplasty. Physiother Can. 2010;62(3):206–14. https://doi.org/10.3138/physio.62.3.206.
Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Jt Surg Am. 2015;97(17):1386–97. https://doi.org/10.2106/JBJS.N.01141.
Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. Lancet. 2012;379(9823):1331–40. https://doi.org/10.1016/S0140-6736(11)60752-6.
Cobelli N, Scharf B, Crisi GM, Hardin J, Santambrogio L. Mediators of the inflammatory response to joint replacement devices. Nat Publ Group. 2011;7(10):600–8. https://doi.org/10.1038/nrrheum.2011.128.
Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28(34):5044–8. https://doi.org/10.1016/j.biomaterials.2007.06.035.
Gurr J-R, Wang ASS, Chen C-H, Jan K-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213(1–2):66–73. https://doi.org/10.1016/j.tox.2005.05.007.
Apostu D, Lucaciu O, Lucaciu GDO, Crisan B, Crisan L, Baciut M, et al. Systemic drugs that influence titanium implant osseointegration. Drug Metab Rev. 2016:1–46. https://doi.org/10.1080/03602532.2016.1277737.
Dörner T, Haas J, Loddenkemper C, von Baehr V, Salama A. Implant-related inflammatory arthritis. Nat Clin Pract Rheumatol. 2006;2(1):53–6. https://doi.org/10.1038/ncprheum0087.
Borghi SM, Mizokami SS, Pinho-Ribeiro FA, Fattori V, Crespigio J, Clemente-Napimoga JT, et al. The flavonoid quercetin inhibits titanium dioxide (TiO2)-induced chronic arthritis in mice. J Nutr Biochem. 2017;53:81–95. https://doi.org/10.1016/j.jnutbio.2017.10.010.
Verri WA, Vicentini FTMC, Baracat MM, Georgetti SR, Cardoso RDR, Cunha TM, et al. Flavonoids as anti-inflammatory and analgesic drugs: mechanisms of action and perspectives in the development of pharmaceutical forms. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry; 2012. pp. 297–330.
Lee CH, Jeong TS, Choi YK, Hyun BH, Oh GT, Kim EH, et al. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun. 2001;284(3):681–8. https://doi.org/10.1006/bbrc.2001.5001.
Sun H, Dong T, Zhang A, Yang J, Yan G, Sakurai T, et al. Pharmacokinetics of hesperetin and naringenin in the Zhi Zhu Wan, a traditional Chinese medicinal formulae, and its pharmacodynamics study. Phytother Res. 2013;27(9):1345–51. https://doi.org/10.1002/ptr.4867.
Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, et al. Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATPChannel signaling pathway. PLoS One. 2016;11(4):e0153015-e. https://doi.org/10.1371/journal.pone.0153015.
Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, Borghi SM, Bordignon J, Silva RL, et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem. 2016;33:8–14. https://doi.org/10.1016/j.jnutbio.2016.03.013.
Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, et al. Naringenin reduces inflammatory pain in mice. Neuropharmacology. 2016;105:508–19. https://doi.org/10.1016/j.neuropharm.2016.02.019.
Wang J, Fan Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: Size, shape, crystal phases, and surface coating. Int J Mol Sci. 2014;15(12):22258–78. https://doi.org/10.3390/ijms151222258.
Ashley NT, Weil ZM, Nelson RJ. Inflammation: mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. https://doi.org/10.1146/annurev-ecolsys-040212-092530.
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. https://doi.org/10.1016/S0140-6736(16)30173-8.
Wang W, Wu C, Tian B, Liu X, Zhai Z, Qu X, et al. The Inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int J Mol Sci. 2014;15(12):21913–34. https://doi.org/10.3390/ijms151221913.
Wang JX, Fan YB, Gao Y, Hu QH, Wang TC. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials. 2009;30(27):4590–600. https://doi.org/10.1016/j.biomaterials.2009.05.008.
Guerrero ATG, Verri WA, Cunha TM, Silva TA, Rocha FAC, Ferreira SH, et al. Hypernociception elicited by tibio-tarsal joint flexion in mice: a novel experimental arthritis model for pharmacological screening. Pharmacol Biochem Behav. 2006;84(2):244–51. https://doi.org/10.1016/j.pbb.2006.05.008.
Yamanaka H, Goto K, Miyamoto K. Scoring evaluation for histopathological features of synovium in patients with rheumatoid arthritis during anti-tumor necrosis factor therapy. Rheumatol Int. 2010;30(3):409–13. https://doi.org/10.1007/s00296-009-1158-2.
Sun J, Hua B, Livingston EW, Taves S, Johansen PB, Hoffman M, et al. Abnormal joint and bone wound healing in hemophilia mice is improved by extending factor IX activity after hemarthrosis. Blood. 2017;129(15):2161–71. https://doi.org/10.1182/blood-2016-08-734053.
Casagrande R, Georgetti SR, Verri WA, Dorta DJ, dos Santos AC, Fonseca MJV. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J Photochem Photobiol B Biol. 2006;84(1):21–7. https://doi.org/10.1016/j.jphotobiol.2006.01.006.
Verri WA, Souto FO, Vieira SM, Almeida SCL, Fukada SY, Xu D, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69(9):1697–703. https://doi.org/10.1136/ard.2009.122655.
Vieira SM, Cunha TM, Franca RF, Pinto LG, Talbot J, Turato WM, et al. Joint NOD2/RIPK2 signaling regulates IL-17 axis and contributes to the development of experimental arthritis. J Immunol. 2012;188(10):5116–22. https://doi.org/10.4049/jimmunol.1004190.
Hohmann MSN, Cardoso RDR, Pinho-Ribeiro FA, Crespigio J, Cunha TM, Alves-Filho JC, et al. 5-lipoxygenase deficiency reduces acetaminophen-induced hepatotoxicity and lethality. BioMed Res Int. 2013;2013:627046-. https://doi.org/10.1155/2013/627046.
Guedes RP, Bosco LD, Teixeira CM, Araújo ASR, Llesuy S, Belló-Klein A, et al. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31(5):603–9. https://doi.org/10.1007/s11064-006-9058-2.
O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. 2016;68(12):2889–900. https://doi.org/10.1002/art.39837.
Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis. 2016;75(6):1187–95. https://doi.org/10.1136/annrheumdis-2014-207137.
Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–9. https://doi.org/10.1038/nri1312.
Liu B, Tai Y, Achanta S, Kaelberer MM, Caceres AI, Shao X, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci. 2016;113(47):E7572–9. https://doi.org/10.1073/pnas.1606608113.
Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor. J Neurosci. 2006;26(1):246–55. https://doi.org/10.1523/JNEUROSCI.3858-05.2006.
Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1 sensors. J Neurosci. 2008;28(52):14062–73. https://doi.org/10.1523/JNEUROSCI.3795-08.2008.
Ebbinghaus M, Segond von Banchet G, Massier J, Gajda M, Brauer R, Kress M, et al. Interleukin-6-dependent influence of nociceptive sensory neurons on antigen-induced arthritis. Arthritis Res Ther. 2015;17:334. https://doi.org/10.1186/s13075-015-0858-0.
Vieira SM, Lemos HP, Grespan R, Napimoga MH, Dal-Secco D, Freitas A, et al. A crucial role for TNF-alpha in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br J Pharmacol. 2009;158(3):779–89. https://doi.org/10.1111/j.1476-5381.2009.00367.x.
Mascarenhas DP, Pereira MS, Manin GZ, Hori JI, Zamboni DS. Interleukin 1 receptor-driven neutrophil recruitment accounts to MyD88-dependent pulmonary clearance of legionella pneumophila infection in vivo. J Infect Dis. 2015;211(2):322–30. https://doi.org/10.1093/infdis/jiu430.
Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–25.
Lan F, Yuan B, Liu T, Luo X, Huang P, Liu Y, et al. Interleukin-33 facilitates neutrophil recruitment and bacterial clearance in S. aureus-caused peritonitis. Mol Immunol. 2016;72:74–80. https://doi.org/10.1016/j.molimm.2016.03.004.
Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16(6):708–12. https://doi.org/10.1038/nm.2156.
Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170(12):6257–65.
Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281(9):5657–67. https://doi.org/10.1074/jbc.M506172200.
Harris WH. Wear and periprosthetic osteolysis the problem. Clin Orthop Relat Res. 2001(393):66–70.
Long M, Rack HJ. Titanium alloys in total joint replacement–a materials science perspective. Biomaterials. 1998;19(18):1621–39.
Chobot V, Hadacek F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011;16(6):242–7. https://doi.org/10.1179/1351000211Y.0000000015.
Straub I, Mohr F, Stab J, Konrad M, Philipp SE, Oberwinkler J, et al. Citrus fruit and fabacea secondary metabolites potently and selectively block TRPM3. Br J Pharmacol. 2013;168(8):1835–50. https://doi.org/10.1111/bph.12076.
Manchope MF, Casagrande R, Verri JWA, Manchope MF, Casagrande R, Verri JWA. Naringenin: an analgesic and anti-inflammatory citrus flavanone. Oncotarget. 2017;8(3):3766–7. https://doi.org/10.18632/oncotarget.14084.
Murakawa M, Yamaoka K, Tanaka Y, Fukuda Y. Involvement of tumor necrosis factor (TNF)-alpha in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem Pharmacol. 2006;71(9):1331–6. https://doi.org/10.1016/j.bcp.2006.01.005.
Seven A, Guzel S, Seymen O, Civelek S, Bolayirli M, Yigit G, et al. Nitric oxide synthase inhibition by L-NAME in streptozotocin induced diabetic rats: impacts on oxidative stress. Tohoku J Exp Med. 2003;199(4):205–10.
Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, et al. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118(4):829–38.
Fattori V, Amaral FA, Verri WA. Neutrophils and arthritis: role in disease and pharmacological perspectives. Pharmacol Res. 2016. https://doi.org/10.1016/j.phrs.2016.01.027.
Scott DL, Wolfe F, Huizinga TWJ. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–108. https://doi.org/10.1016/S0140-6736(10)60826-4.
Kongpichitchoke T, Hsu J-L, Huang T-C. Number of hydroxyl groups on the B-Ring of flavonoids affects their antioxidant activity and interaction with phorbol ester binding site of PKCδ C1B domain: in vitro and in silico studies. J Agric Food Chem. 2015;63(18):4580–6. https://doi.org/10.1021/acs.jafc.5b00312.
Wang CC, Guo L, Tian FD, An N, Luo L, Hao RH, et al. Naringenin regulates production of matrix metalloproteinases in the knee-joint and primary cultured articular chondrocytes and alleviates pain in rat osteoarthritis model. Braz J Med Biol Res. 2017;50(4):e5714. https://doi.org/10.1590/1414-431X20165714.
Murphy G, Lee MH. What are the roles of metalloproteinases in cartilage and bone damage? Ann Rheum Dis. 2005;64(Suppl 4):iv44–7. https://doi.org/10.1136/ard.2005.042465.
Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis Cartilage. 2004;12(6):485–96. https://doi.org/10.1016/j.joca.2004.02.012.
Steinert AF, Noth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury. 2008;39(Suppl 1):97–113. https://doi.org/10.1016/j.injury.2008.01.034.
Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292(4):490-. https://doi.org/10.1001/jama.292.4.490.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. https://doi.org/10.1038/nature01658.
Chakravarti A, Raquil M-A, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4–stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114(8).
Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, et al. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 2015;37(10):924–33. https://doi.org/10.1179/1743132815Y.0000000079.
Nishimura FdCY, de Almeida AC, Ratti BA, Ueda-Nakamura T, Nakamura CV, Ximenes VF, et al. Antioxidant effects of quercetin and naringenin are associated with impaired neutrophil microbicidal activity. Evid Based Complement Alternat Med. 2013;2013:795916-. https://doi.org/10.1155/2013/795916.
Gonçalves DM, Chiasson S, Girard D. Activation of human neutrophils by titanium dioxide (TiO2) nanoparticles. Toxicol In Vitro. 2010;24(3):1002–8. https://doi.org/10.1016/j.tiv.2009.12.007.
Masoud R, Bizouarn T, Trepout S, Wien F, Baciou L, Marco S, et al. Titanium dioxide nanoparticles increase superoxide anion production by acting on NADPH oxidase. PLoS One. 2015;10(12):e0144829-e. https://doi.org/10.1371/journal.pone.0144829.
Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–70. https://doi.org/10.1007/s00774-015-0656-4.
Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW, et al. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem. 2010;285(10):6913–21. https://doi.org/10.1074/jbc.M109.051557.
Rees MD, Hawkins CL, Davies MJ. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem J. 2004;381(Pt 1):175–84. https://doi.org/10.1042/BJ20040148.
Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747–55.
Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C, Miyazaki T, et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res. 2011;26(11):2682–94. https://doi.org/10.1002/jbmr.489.
Kang IS, Kim C. NADPH oxidase gp91(phox) contributes to RANKL-induced osteoclast differentiation by upregulating NFATc1. Sci Rep. 2016;6:38014. https://doi.org/10.1038/srep38014.
Pinho-Ribeiro FA, Fattori V, Zarpelon AC, Borghi SM, Staurengo-Ferrari L, Carvalho TT, et al. Pyrrolidine dithiocarbamate inhibits superoxide anion-induced pain and inflammation in the paw skin and spinal cord by targeting NF-κB and oxidative stress. Inflammopharmacology. 2016;24(2–3):97–107. https://doi.org/10.1007/s10787-016-0266-3.
St Pierre CA, Chan M, Iwakura Y, Ayers DC, Kurt-Jones EA, Finberg RW. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res. 2010;28(11):1418–24. https://doi.org/10.1002/jor.21149.
Borgognoni CF, Mormann M, Qu Y, Schäfer M, Langer K, Öztürk C, et al. Reaction of human macrophages on protein corona covered TiO2 nanoparticles. Nanomedicine: nanotechnology, biology, and medicine. 2015;11(2):275–82. https://doi.org/10.1016/j.nano.2014.10.001.
Vitkov L, Krautgartner W-D, Obermayer A, Stoiber W, Hannig M, Klappacher M, et al. The initial inflammatory response to bioactive implants is characterized by NETosis. PLoS One. 2015;10(3):e0121359-e. https://doi.org/10.1371/journal.pone.0121359.
Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(10):593–601. https://doi.org/10.1038/nrrheum.2014.80.
Freischmidt A, Jürgenliemk G, Kraus B, Okpanyi SN, Müller J, Kelber O, et al. Contribution of flavonoids and catechol to the reduction of ICAM-1 expression in endothelial cells by a standardised Willow bark extract. Phytomedicine. 2012;19(3–4):245–52. https://doi.org/10.1016/j.phymed.2011.08.065.
Martinelli R, Gegg M, Longbottom R, Adamson P, Turowski P, Greenwood J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol Biol Cell. 2009;20(3):995–1005. https://doi.org/10.1091/mbc.E08-06-0636.
Dal Secco D, Moreira AP, Freitas A, Silva JS, Rossi MA, Ferreira SH, et al. Nitric oxide inhibits neutrophil migration by a mechanism dependent on ICAM-1: role of soluble guanylate cyclase. Nitric Oxide. 2006;15(1):77–86. https://doi.org/10.1016/j.niox.2006.02.004.
Kevil CG, Patel RP, Bullard DC. Essential role of ICAM-1 in mediating monocyte adhesion to aortic endothelial cells. Am J Physiol Cell Physiol. 2001;281(5):C1442-7.
Verri WA, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112(1):116–38. https://doi.org/10.1016/j.pharmthera.2006.04.001.
Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF- B and its relevance to arthritis and inflammation. Rheumatology. 2008;47(5):584–90. https://doi.org/10.1093/rheumatology/kem298.
Yu M, Qi X, Moreno JL, Farber DL, Keegan AD. NF-κB signaling participates in both RANKL- and IL-4-induced macrophage fusion: receptor cross-talk leads to alterations in NF-κB pathways. J Immunol. 2011;187(4):1797–806. https://doi.org/10.4049/jimmunol.1002628.
Meena R, Kumar S, Paulraj R. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J Nanopart Res. 2015;17(1):49-. https://doi.org/10.1007/s11051-015-2868-x.
Prasad RY, Simmons SO, Killius MG, Zucker RM, Kligerman AD, Blackman CF, et al. Cellular interactions and biological responses to titanium dioxide nanoparticles in HepG2 and BEAS-2B cells: role of cell culture media. Environ Mol Mutagen. 2014;55(4):336–42. https://doi.org/10.1002/em.21848.
Acknowledgements
We thank M.R.F.D.P., A.Z.Z., and M.M.B for the technical support and T.H.Z for the support in the Adobe Illustrator. This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), São Paulo research Foundation (FAPESP) under grant agreements number 2011/19670-0 (Thematic project) and 2013/08216-2 (Center for Research in Inflammatory Disease-CRID), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001), and Programa de Pesquisa para o SUS (PPSUS) grant supported by Ministério da Ciência, Tecnologia e Inovação (MCTI), Secretaria da Ciência, Tecnologia e Ensino Superior (SETI), Decit/SCTIE/MS through CNPq with the support of Fundação Araucária and Secretaria da Saúde do Estado do Paraná (SESA-PR), and Parana State Government (Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Jason J. McDougall.
Rights and permissions
About this article
Cite this article
Manchope, M.F., Artero, N.A., Fattori, V. et al. Naringenin mitigates titanium dioxide (TiO2)-induced chronic arthritis in mice: role of oxidative stress, cytokines, and NFκB. Inflamm. Res. 67, 997–1012 (2018). https://doi.org/10.1007/s00011-018-1195-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1195-y