Abstract
Introduction
Apoptotic death of different cells observed during infection is thought to limit overwhelming inflammation in response to microbial challenge. However, the underlying apoptotic death mechanisms have not been well defined. Tumor necrosis factor (TNF) related apoptosis-inducing ligand (TRAIL) is a type II transmembrane protein belonging to the TNF superfamily, which is involved not only in tumor growth suppression but in infection control and also in the regulation of both innate and adaptive immune responses.
Findings
In this review, we have summarized data of recent studies on the influence of the TRAIL/TRAIL receptor (TRAIL-R) system on the development of viral and bacterial infections. TRAIL may have a dual function in the immune system being able to kill infected cells and also to participate in the pathogenesis of multiple infections. Moreover, many pathogens have evolved mechanisms to manipulate TRAIL signaling thus increasing pathogen replication.
Conclusion
Present data highlight an essential role for the TRAIL/TRAIL-R system in the regulation and modulation of apoptosis and show that TRAIL has distinct roles in pathogenesis and pathogen elimination. Knowledge of the factors that determine whether TRAIL is helpful or harmful supposes its potential therapeutic implications that are only beginning to be explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
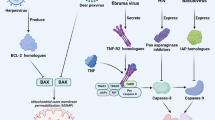
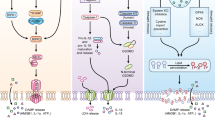
Apoptosis participates in very divergent processes such as the elimination of autoreactive T and B cells, the destruction of infected cells, and the keeping under control the excessive immune response after infection has been overwhelmed [1]. The TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) belongs to the tumor necrosis factor (TNF) family that is responsible for extrinsic induction of cell death. TRAIL is composed of an extracellular TNF-like domain, an extracellular stalk, a transmembrane helix, and a small cytoplasmatic part. After breaking at the stalk domain TRAIL becomes a soluble molecule able to interact with other two molecules at position 230 (Cys230). The TRAIL/TRAIL-R interactions might have immunosuppressive or immunoregulatory functions, as well as they might play proviral or antiviral, and tumour immunosurveillance roles [2, 3]. Imbalance in apoptotic processes can result in serious cancer and autoimmune diseases. The induction of apoptosis is a result of the binding of TRAIL to two death receptors TRAIL-R1 (DR4) or TRAIL-R2 (DR5). TRAIL can bind and to two non-apoptosis-inducing receptors named TRAIL-R3 (LIT, DcR1) and TRAIL-R4 (TRUNDD, DcR2), also called decoy receptors. Additionally, a soluble receptor osteoprotegerin (OPG) is capable of interaction with TRAIL. Thereby, the receptors are trimerised and the death-inducing signalling complex (DISC) is formed [4]. TRAIL binding to its specific death receptors can cause another form of programmed cell death named necroptosis which plays an important role as a viral defense mechanism, allowing the cell to undergo cellular death in a caspase-independent fashion [5, 6]. In mice, there is one full-length receptor, TRAIL-R (mDR5, mTRAILR2), which is equally homologous to human DR4 and DR5 [7, 8]. Also, two murine decoy receptors mDcTRAILR1 and mDcTRAILR2 which lack a death domain have been identified [9]. Both, TRAIL and TRAIL-Rs are constitutively expressed in various tissues [10, 11] and are up-regulated upon cell activation [12, 13]. TRAIL has the ability to induce apoptosis preferentially in transformed cells such as tumor cells, while in nontransformed cells the actions of TRAIL are less characterized [14]. Subsequent studies have shown that binding of TRAIL to TRAIL-R1 and TRAIL-R2 can activate a number of factors, including NFκB, PI3K/Akt, MAPKs, ERK1/ERK2, JNK and p38, resulting in generation of survival signals [15, 16]. Besides its involvement in tumor growth and elimination of infected cells, TRAIL participates in the regulation of immune system balance. TRAIL is expressed by various cells of the immune system, including natural killer (NK) cells [17], activated T cells [13], natural killer T cells (NKT cells), dendritic cells [18] and monocyte/macrophages [19]. In a model of autoimmune lymphoproliferative syndrome (ALPS), mice deficient in both FasL and TRAIL show lymphadenopathy, splenomegaly and accumulation of double-negative CD4-CD8- T cells [20]. Except, TRAIL-deficient mice are predisposed to develop collagen-induced arthritis and streptozotocin-induced diabetes, associated with impaired thymocyte apoptosis. Thus, TRAIL contributes to the regulation of central tolerance through a decrease of autoreactive thymocytes [21] and also by enhancement the proliferation of T regulatory cells [22]. TRAIL can affect adaptive immunity as a consequence of inhibited T cell activation and expansion. TRAIL-deficient mice show a greater frequency of CD4+ Th1 cells than do wild-type mice due to the suppression of apoptosis [22, 23], while CD8+ antigen-activated T cells are eliminated by TRAIL-mediated mechanisms [24].
It is clear from the studies in animals and humans with infection, that this is a condition having a very complicated character. TRAIL can provoke an immune suppression through inducing a massive death of different immune cell populations. If a new infection challenge appears, the TRAIL-dependent suppressive mechanisms will prevent pathogen clearance and the animal/man is sensitive to the infection. Blocking TRAIL through systemic anti-TRAIL mAb administration, prevents the pathogen-induced immune suppression, keeping entire T cell-mediated immune function to clear the infection, and increase the survival. It was demonstrated that the inoculation of TRAIL-deficient mice with different pathogens or challenge with Toll like receptor (TLR) agonists improve their ability to clear the infection, due to the increased production of pro-inflammatory-cytokines such as interleukine (IL)-12 and interferons (IFNs) [25]. Yet, TLRs 7, 8 and 9 belong to the endosomal receptors that recognize single-stranded RNA derived from viruses [26]. Moreover, some viral proteins and ribonucleoprotein (RNP) complexes are also capable to trigger IFN signaling and generate antiviral response against most viruses, particularly by plasmacytoid dendritic cells (pDC) and bone-marrow derived DCs [27, 28]. In that way, herpes simplex virus (HSV)-1, HSV-2, and murine cytomegalovirus (MCMV) infections are accompanied with high levels of type I IFN production in pDCs [29–31]. The right way to eliminate the infection is through tightly controlled inflammation, as excessive inflammation can enhance pathology. The question of how endogenous TRAIL participates in immune homeostasis is in the focus of many recent investigations. The answers will help in finding of new therapeutic approaches.
TRAIL in viral infections
Viral infection usually causes inflammatory reaction characterized by release of cytokines and chemokines, and affects many cellular processes such as cell signaling, apoptosis, transcription and DNA repair. Various viral proteins have been shown to induce apoptosis or autophagy on their own, independent of the presence of other viral proteins. Type I IFNs are produced to high levels during viral infections and they are essential players in innate and adaptive immunity against viruses [32, 33]. Mice lacking functional IFNαβR are more sensitive to vaccinia virus, lymphocytic choriomeningitis virus and vesicular stomatitis virus [34]. A number of investigations have been devoted to the importance of IFNαβ for the development and exit of influenza virus infection [35]. High IFNαβ levels strongly exacerbate the disease while IFNαβ receptor deficiency lowered morbidity and mortality [36–38]. TLRs are key transducers of type I IFN signaling after recognition of certain viral components. TLR3 and TLR4 recognize viral dsRNA leading to the activation of NFκB [39, 40]. Also, dsDNA and capsid components of the human papiloma virus (HPV) are detected by TLRs contributing to HPV regression [41]. Many investigations witness that non-infected cells are resistant to TRAIL-induced apoptosis, while they become sensitive in a result of viral infections [42–44]. The antiviral response against encephalomyocarditis virus (ECMV) depends on the NK cells expressing TRAIL, controlled by IFNαβ produced by virus-infected cells. Neutralizing of secreted TRAIL increases viral titers and causes earlier death of infected mice [44]. Susceptible 129 mice are highly protected from influenza infection when plasmacytoid dendritic cells expressing PDCA-1 marker have been deleted. This is in correlation with high IFNαβ production by these cells and with an elevated expression of TRAIL on inflammatory monocytes and DR5 expression on epithelial cells. The impairment of TRAIL-DR5 interaction prevents lung tissue damage [36].
Influenza virus-infected cells undergo apoptosis and become susceptible to phagocytosis by macrophages that leads to inhibited virus replication. Additionally, the control of primary influenza infections depends to a great extent on CD8+ T cells which eliminate virus-infected cells through perforin-, FasL- and TRAIL-mediated mechanisms. The role of TRAIL-induced apoptosis is demonstrated by data showing that adoptive transfer of TRAIL+/+ but not TRAIL−/− CD8+ effector T cells decreased the mortality associated with lethal dose influenza virus infections [45]. In a murine model of influenza virus pneumonia, lung-originated macrophages provoke alveolar epithelial cell apoptosis through an elevated release of TRAIL, further leading to increased mortality. Accordingly, the inhibition of TRAIL signaling in alveolar macrophages reduces the apoptosis of alveolar epithelial cells that resulted in an increased survival rate [46].
Hepatitis C virus (HCV) infection is one of the established causes of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Effective virus elimination requires the combined function of different arms of the immune system, including those belonging to the innate immune system, such as IFNs, NK and NK T cells, as well as the adaptive immune response specific to a given pathogen generated by CD4+ and CD8+ T cells. Dendritic cells play a central role in antiviral immunity as it stands in HCV infection, where a subset of human DCs displays direct cytotoxic activity. CD1c-expressing myeloid DCs cause apoptosis of activated T cells and subsequent down-regulation of antiviral immune responses via TRAIL. In patients developing chronic hepatitis lysis of target cells is not observed [47]. NK cells from patients with chronic hepatitis C are able to induce apoptosis of hepatic stellate cells. This effect has been most eminent if the patients have been treated with IFN-α. The anti-fibrotic role of NK cells correlates with upregulated expression of TRAIL [48]. Chronic liver inflammation caused by HCV infection leads to hepatocellular carcinoma and liver failure. Virus activated caspase-8 through DR4 and DR5 and the apoptosis of infected cells is enhanced in the presence of rTRAIL [49]. In patients with hepatitis B virus (HBV) infection an increase of TRAIL expression in the liver has been established [50]. Although, TRAIL participates in the immune response against HBV no strict correlation with the rate of liver injury has been found. A strong elevation of TRAIL, TRAIL-R1 and TRAIL-R2 in respiratory syncytial virus (RSV) infection has been observed in correlation with apoptosis of RSV-infected cells [51]. Neutrophils are major effector cells that limit cytomegalovirus (CMV) infection through elimination of infected cells, realizing their antiviral activity via TRAIL. Neutrophil recruitment to peripheral tissues is a result of increased IL-22-R expression on their surface followed by enhanced TRAIL-R expression on infected cells and probably attended with increased TRAIL production [52]. Thus, neutrophils represent important participants in antiviral control due to TRAIL/TRAIL-R interaction. In contrast, in a model of respiratory vaccinia virus infection in mice Goulding et al. [53] have demonstrated that TRAIL-dependant pathways are not involved in the protective mechanisms against virus [53]. These results prove the meaning of the type of pathogen for the overall TRAIL/TRAIL-R effects.
A variety of virus infections have been associated with apoptotic cell death as a defense mechanism leading to the elimination of infected cells. On the other hand, the inhibition of apoptosis by several viruses may prevent the death of infected cells, thus augmenting viral replication and spread. The apoptosis of infected cells may be also induced by CTLs and NK cells or by the action of different cytokines. For example, apoptosis proved to be one of the major mechanisms responsible for the pathology and outcome in dengue infection. Arias et al. have aimed to find a correlation between serum levels of several cytokines including TNF-α, IL-6, IL-1, IL-17, sST2, sTRAIL, IL-12 and TNF-Rs with the severity of disease. In general, sTRAIL is clearly involved in dengue pathology and its elevation in sera pointed that it might be used as a disease biomarker. However, a beneficial effect of sTRAIL is supposed in the early phase of infection, while in severe disease it might be injurious [54]. Macrophages/monocyte populations from infected patients produce high amounts of sRANKL and it might explain the enhanced monocyte apoptosis. On the other hand, in vitro cultivation of primary human monocytes, B cells and DCs in the presence of dengue virus results in high sTRAIL release [55].
The elimination of human immunodeficiency virus type I (HIV-1) infected cells through TRAIL-induced apoptosis is another example how apoptosis can contribute to disease pathogenesis. The virus causes the production of type I IFNs by pDCs, which provokes the expression of membrane-bound TRAIL on CD4+ T cells and TRAIL production by monocytes. Type I IFN-regulated TRAIL/DR5 mechanisms lead to apoptosis of HIV-1-exposed CD4+ T cells. The decrease of viral load and the increase of CD4 counts in HIV-1 patients are associated with a decrease in expression of DR5 mRNA by CD4+ T lymphocytes [56]. In HIV patients subjected to highly active antiretroviral therapy the rate of apoptosis of PBMCs and CD8 T lymphocytes remains high. The increased T cell apoptosis and the impaired CD4 recovery can prevent immune restoration [57]. The number of apoptotic CD4+ T cells expressing DR5 is higher in infected patients compared to uninfected controls. Infectious and noninfectious HIV-1 virions provoke high expression of TRAIL and DR5 by CD4+ cells only, but not by CD8+ T cells. Moreover, the use of anti-DR5 antibodies decreased the apoptosis of CD4+ T cells isolated from HIV patients [56]. A number of HIV-1 accessory proteins such as Vpr, Vpu, Nef, Vif, Rev and Tat induce apoptosis in virus infected cells and moreover, they also can promote apoptosis in uninfected bystander cells [58]. Vpr-mediated apoptosis is associated with an activation of caspase-9 and capase-3 [59]. Vpr can exert dual roles in respect to apoptotic processes which is related to the intracellular Vpr expression at different stages of HIV-1 infection, resulting in either viral persistence or virus dissemination. Vpu and Vif cause CD4+ depletion leading to impaired immune response and to promoting the release of infectious virions [60]. Massive apoptosis of uninfected bystander T cells mediated by another HIV-1 accessory Tat 1 protein was observed. Thus, through Tat 1 reduction the severity of the infection might be ameliorated [61]. The HIV-1 protein Nef is an essential modulator of AIDS pathogenesis. Nef activates T cells in a variety of experimental models making them highly susceptible to apoptosis. This is a result of reduced expression of the anti-apoptotic proteins Bcl-2 and Bcl-X(L) and followed by enhancement of apoptotic death [62].
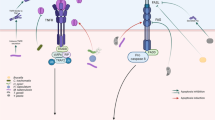
Autophagy is another way to efficiently eliminate invading bacterial and viral pathogens. Shift to autophagy or to TRAIL-induced apoptosis depends on the caspase-8 activity and the activation of the mitochondrial apoptotic pathway [63, 64]. In a model of Sindbis viral infection the overexpression of the ATG protein beclin-1 resultes in decreased viral replication and increased survival [65]. The autophagy causes the degradation of HSV-1 virions and viral particles in autophagosomes and influences viral replication [66], followed by TLR recognition and production of type I IFNs. Autophagy is related to the presentation of viral proteins on MHC I and MHC II [67]. Coxsackieviruses (CVB) and poliovirus induce in vitro autophagy upon infection and substantionally augment viral replication [68, 69]. In the case of poliovirus, autophagy operate in endoplasmic reticulum and lead to the delivery of viral proteins 2BC and 3A, which anchor to cellular membranes during poliovirus RNA synthesis. Also, in dengue virus infection autophagy in infected cells contributes to strong viral replication [70]. However, the course of infection will depend on the ability of certain virus to inhibit autophagy or oppositely, to ensure maximal viral replication.
In the in vitro experiments Clarke et al. have shown that reovirus induced an apoptosis of infected HEK293 cells after TRAIL binding to both DR5 and DR4 receptors, while uninfected cells have not been triggered to apoptosis [42]. The authors claim that it is due to insufficient cell surface DR4 or DR5 on uninfected cells. So, the pathogen up-regulates TRAIL release and the expression of death-associated TRAIL receptors. This increase is mainly due to DR5 elevation compared to DR4, thus DR5 seems to be more involved in apoptotic cell death. Furthermore, in vitro investigations show that RSV-infected cells release TRAIL and also express membrane TRAIL and its functional receptors DR4 and DR5. RSV-infected cells become highly sensitive to apoptosis induced by exogenous TRAIL [71].
TRAIL in bacterial infections
Apoptosis is a defense mechanism involving both innate and adaptive immunity although pathogenic organisms can modulate apoptosis for their survival. In some cases apoptosis of the infected cells results in dissemination of infections like Yersinia and Francisella [72, 73], or the impairment of apoptosis provides a survival for many intracellular pathogens including Mycobacterium tuberculosis [74]. Gastrointestinal gram-negative bacterial pathogens such as Salmonella, Shigella and enteropathogenic Escherichia coli colonize the gut, replicate in host tissues and cause successful infection. They liberate a number of effector proteins to turn host signal transductions for escape of their clearance [75, 76]. Autophagy can function as either a promoter or a suppressor of infection, which makes it an interesting therapeutic target. Some intracellular pathogens such as L. monocytogenes can avoid phagosomes and thus it can replicate in the cytoplasm. If autophagic mechanisms are activated the bacteria are degraded in endocytic and lysosomal compartments [77]. Mycobacterium tuberculosis although can escape fusion to lysosomes it can induce autophagy, leading to the degradation of this pathogen [78].
Staphylococcus aureus is a leading cause of bacteremia as well as of osteoarticular, skin and soft tissue and pleuropulmonary disorders. Osteomyelitis (OM) is a consequence of hematogenous spread or direct bone inoculation of bacteria leading to acute or chronic infection [79]. Activation of TLRs in osteoblasts induces the production of osteoclastogenic cytokines, such as RANKL and TNF-α, thereby contributing to TLR ligand-induced osteoclastogenesis. These processes are the reason for the bone loss observed in variety of infectious diseases. In adults, Staphylococcus aureus is the most common pathogen in OM [80]. Osteoblasts are supposed to be directly infected with S. aureus, nevertheless being extracellular pathogen [81]. In such a way osteoblasts become a source of maintenance the infection. Along with this, infected osteoblasts produce TRAIL which can drive bone remodeling toward resorption as a result of osteoblast apoptosis [82, 83]. Mycobacterium avium infection in mice is an appropriate model to study the control of T cells by apoptosis, because it represents a chronic infection with more than 40 weeks duration. Apoptosis of both CD4+ and CD8+ T cells, increased with the progress of infection that helps to avoid immunopathology [84]. TRAIL deficient mice have been used to study its role in Mycobacterium avium infection. During the disease it has been found an up-regulation of caspase-8 activity in spleen and strong lymphocyte depletion in secondary lymphoid organs. TRAIL−/− mice did not show an increase of survival and developed splenic lymphopenia in disseminated infection or granuloma necrosis during low-dose infection [85].
Helicobacter pylori infection is associated with a wide range of upper gastrointestinal diseases such as gastritis, peptic ulcer disease and gastric cancer. Histopathologic examination of the infected stomach has shown that more apoptotic epithelial cells have been found than in normal [86]. The results showing that the number of apoptotic epithelial cells decrease to normal after elimination of H pylori, prove that the bacterium is responsible for the increased apoptosis. It is known that human gastric epithelial cells are resistant to TRAIL-mediated apoptosis while the apoptosis of these cells arises during H. pylori infection [87, 88]. The application of a soluble TRAIL receptor could specifically block the TRAIL-mediated apoptosis in vitro. The infiltrating T-cells in gastric mucosa express TRAIL on their surfaces, and the pathogen causes TRAIL sensitivity upon direct cell contact with viable bacteria [8]. The mechanism of breaking the resistance to TRAIL apoptosis is well described by Lin et al. [89]. By regulation of cellular FLICE-inhibitory proteins (c-FLIP) and formation of a death-inducing signaling complex (DISC) initiates caspase activation and triggers the caspase cascade.
Apoptosis plays a critical role also in the pathogenesis of Listeria monocytogenes (L. monocytogenes) [90]. Mice deficient in TRAIL are more resistant to primary infection and tissue injuries are less expressed. Blocking TRAIL action with anti-DR5 antibodies ameliorates cell death and enhances host defense against Listeria infection. The amount of bacteria recovered from the liver and spleen of TRAIL−/− mice have been remarkably reduced compared to those of TRAIL+/+ mice, although the inhibited cell death of lymphoid and myeloid cells has lead to a spleen enlargement. Interesting results were reported by Gurung et al. in TRAIL−/− and DR5−/− mice [91]. They have performed a combined model of sepsis including secondary infection with ovalbumin (OVA)-257-expressing L. monocytogenes (LM-OVA) subsequent to cecal-ligation and puncture (CLP). Deficient mice have been better protected from secondary infection, as well as the administration of a blocking anti-TRAIL mAb to CLP-treated WT mice ameliorates LM-OVA infection. TRAIL-induced immune suppression is related to the reduced bacterial Ag-specific CD8+ T cell response. These results point on the TRAIL-mediated immune suppression during sepsis and suppose that TRAIL neutralization may be a promising therapeutic target to restore cellular immunity in septic patients.
TRAIL/TRAIL-R pathway is firstly studied in cancer and the results have shown its regulatory role in metastasis formation in human tumors. TRAIL−/− and TRAILR−/− deficient mice have been used in various infectious models. Diehl et al. have investigated comparatively some viral and bacterial infections in TRAIL-R−/− mice that have shown different results in respect to the type of infection and the type of response [25]. TRAIL-R deficiency lead to enhanced resistance against murine cytomegalovirus (MCMV) challenge and increased cytokine production in Bacille Calmette–Guérin (BCG)-infected macrophages. No apparent resistance against L. monocytogenes, Salmonella typhimurium and Mycobacterium bovis has been established. Macrophages and dendritic cells lacking TRAIL-R produce increased cytokine levels after an ex vivo treatment with some TLR stimuli. Clearing of Chlamydia trachomatis infection is associated with strong inflammatory response generated by the host. This response in many cases can cause chronic inflammation and tissue damage [92]. The increased expression of TRAIL-R1 has been found indicative for the course of infection. TRAIL-R1 might be considered as a negative regulator of inflammation and plays a potential role in modulating pathogenesis [93]. The processes of apoptosis are important part of Chlamydia muridarum (trachomatis) lung infection through IFN-dependant mechanisms. IFNα-R−/− mice show lower expressions of IFN I-induced apoptotic factors, such as TRAIL, death-domain associated protein (Daxx), and protein kinase R (PKR) resulting in inhibited macrophage apoptosis [94]. In this case, the presence of IFNIs dose not improve the course of C. muridarum infection in normal mice while in IFNαR−/− mice less contamination and less injury of the lungs have been observed. In deficient mice the infiltration of macrophages into the lungs has been increased, but the macrophages have not been removed by apoptotic processes. It supposes the correlation between the number of macrophages and bacterial clearance especially in the early stage of infection.
Apoptosis is observed during pneumococcal meningitis and pneumonia. Epithelial cell apoptosis contributes to tissue injury but the lung inflammation caused by Streptococcus pneumoniae infection might be overwhelmed by apoptotic death of infected alveolar macrophages [95]. TRAIL−/− mice show weak bacterial clearance of S. pneumoniae and higher lethality. These effects have been accompanied with significantly diminished apoptosis and caspase-3 activation, and a shift towards necrosis. In wild type mice, TRAIL is produced mainly by neutrophils and their depletion results in necrosis instead of apoptosis. The administration of anti-DR5 mAb (MD5-1) profoundly improves the survival of wild type mice. As well as, the treatment of neutropenic mice with DR5 mAB increases the survival of these mice lacking neutrophil-derived TRAIL. In a model of experimental meningitis provoked by intrathecal application of pneumococcal cell wall it has been observed that TRAIL may act as a negative regulator of acute central nervous system inflammation [96]. Studies in TRAIL−/− mice clearly demonstrate that TRAIL deficiency extends the duration and promotes exacerbation of inflammation, along with the increase of hippocampal cell apoptosis [96]. Moreover, intrathecal application of rTRAIL in TRAIL−/− reverses these effects and in wild-type mice with meningitis it decreases inflammation and apoptosis. TRAIL application attenuates injurious changes as well as such effect has been achieved by the transplantation of TRAIL-expressing BM cells. The experiments in TRAIL−/− mice have shown delayed onset of inflammation along with prolonged survival of activated CSF cells. The influx of neutrophils has been decreased leading to an impaired bacterial clearance. In patients with bacterial meningitis an elevated serum TRAIL levels have been observed. These data point on a new approach for clinical application of TRAIL in bacterial meningitis based on the increased intrathecal synthesis of TRAIL, observed in patients with meningitis. In murine polymicrobial intraabdominal sepsis (colon ascendens stent peritonitis, CASP) TRAIL exhibits strong protective effect demonstrated by increased survival [97]. The results have shown that this beneficial effect is associated with enhanced apoptosis of neutrophils in spleen, lung and liver, and that they express DR5 at high density. In the case, when neutrophils have been depleted prior to sepsis induction TRAIL fails to prevent organ injury. Table 1 presents some TRAIL-dependant pathologic effects in bacterial infections.
To these in vivo results should be added in vitro data obtained by Leverkus et al. [98]. The authors have proved that primary keratinocytes are less sensitive to TRAIL-induced apoptosis than transformed keratinocytes. This difference is due to the intracellular inhibition of the TRAIL death part of TRAIL-R1 and TRAIL-R2 rather than to both decoy receptors TRAIL-R3 and TRAIL-R4. Also, TRAIL induces apoptosis in IFN-γ stimulated keratinocytes, particularly in proliferating but not in differentiating human keratinocytes [99]. Additionally, TRAIL is capable to induce caspase-dependant differentiation of primary keratinocytes. Complementary in vitro and in vivo experiments are therefore essential to better understand the seemingly contradictory role of TRAIL in different infections.
Discussion
The pathogens influence on host cell signaling through release of virulence proteins further directly translocated into the host cell cytoplasm and triggering various signaling pathways. Death receptors such as TNFR1, FAS and TRAIL-R unlock signaling events that are vital for the clearance of pathogens, and as such are major targets for inhibition by pathogens. TRAIL is expressed in a variety of human tissues, in particular in the lymphoid system, suggesting an important role in the innate and adaptive immunity. Apoptosis is responsible for the physiological removal of damaged or senescent cells, in mature tissues, as well as in tissue remodeling during development. By counterbalancing mitosis, apoptosis is important to keep tissue homeostasis during normal cell turnover, and to control tissue growth and regeneration. Localized in the plasma membrane, TRAIL-R1 and TRAIL-R2, induce apoptosis and non-apoptotic signaling when crosslinked by the ligand TRAIL or by agonistic receptor-specific antibodies. In addition, these receptors are also found in noncanonical intracellular locations such as autophagosomes, in cytosolic compartment as well as in the nucleus (Fig. 1). The intracellular TRAIL locations represent an additional possibility to extend the list of nucleic acid targets which have not yet been exploited. Host cell apoptosis has been identified as a protective mechanism in various viral and bacterial infection models, as targeted apoptosis induction in infected host cells hinders the spread of bacterial and viral pathogens [74]. The increasing number of recent data in the field of TRAIL-mediated signaling has extended our current information about inducing and maximizing TRAIL-induced apoptosis in infectious processes. TRAIL may perform dual role with available data suggesting promotion of innate antiviral response and contribution to the detrimental effects of viral infection. First one, is its antiinfectious mission to kill virally infected cells and another one, is to participate in the pathogenesis of viral infections. Different viruses affect TRAIL and TRAIL-R expression in a different manner. Reovirus and human cytomegalovirus infections up-regulate TRAIL, TRAIL-R1, and TRAIL-R2 expression on infected cells that result in a remarkable apoptosis [42, 100]. The contradictory role of TRAIL in viral infections raise the question, what factors determine the switch between pro- and anti-inflammatory functions of TRAIL. Having in view the adaptive potential of viruses, their ability to modulate the metabolism, and to control the survival of the infected cells, we need to further understand how the specific viral proteins affect the pathways to apoptosis and autophagy. Thus, we will be able to minimize the pathology that they cause and to look for new therapeutic strategies. In Table 2 different types of cells involved in TRAIL-related processes are shown. Except protective, TRAIL-mediated apoptosis might cause chronic inflammation leading to tissue injury (Fig. 1). During the development of hyperinflammation the role of TRAIL depends on the stage of disease. For example, in sepsis the protective effect of TRAIL is observed at the onset of inflammation associated with the apoptosis of activated neutrophils, while the late phase of sepsis is characterized by immune suppression. Bad role of TRAIL is illustrated by the enhanced apoptosis of gastric epithelial cells in Helicobacter pylori infection. The pathogen not only directly triggered apoptosis of gastric cells but also induced sensitivity to TRAIL-mediated apoptosis through activation of caspase 8 downstream pathway [101]. The expression of proapoptotic receptors TRAIL-R1 and TRAIL-R2 was down-regulated, whereas the expression of antiapoptotic receptors TRAIL-R3 and TRAIL-R4 was up-regulated. TRAIL and TRAIL-Rs are involved in the pathogenesis of H. pylori infection as they may attenuate exaggerated apoptosis and thus limiting tissue injury. At the same time, the pathogen instead of being cleared may keep a niche for its surviving (Fig. 1). Importantly, TRAIL except apoptotic cell death can provoke non-apoptotic death through autophagy or necrosis.
In mouse models it has been observed that TRAIL caused apoptosis of tumor cells with low hepatotoxicity [102]. Experiments in TRAIL receptor-selective mutants showed that DR5 is to a great extent involved in TRAIL-mediate apoptosis rather than DR4 [103]. Currently, two categories of pharmacological agents are clinically applied in cancer therapy, such as recombinant forms of TRAIL and agonistic antibodies against TRAIL-R1 or TRAIL-R2. However, TRAIL might also bind non-death TRAIL receptors, which could prevent its apoptotic activity. It has been established that recombinant forms of human TRAIL are cleared within hours of systemic application, while the half-life of therapeutic antibodies is in the range of several days to weeks. Dulanermin is the only form of recombinant TRAIL tested in phase-I clinical trials for cancer therapy, possessing no toxicity [7]. Mapatumumab is a TRAIL-R1 agonist and conatumumab, lexatumumab, tigatuzumab and drozitumab are TRAIL-R2 agonists which have reached clinical trials [104–106], TRAIL was fused to the Fc portion of human IgG1 resulting in a prolonged half-live in vivo [107]. These promising results in cancer therapy prompt the ways for the application of TRAIL and TRAIL agonists or antagonists in cases of viral or bacterial infections.
Conclusion
TRAIL plays a significant role in the complex interplay between pathogens and their hosts and functions as either a pro- or anti-infectious factor depending on the microorganism and also on host cell type involved. Further investigations are needed to define the cell populations expressing TRAIL consistently and to accumulate more data on the role of decoy receptors as TRAIL-R3 and TRAIL-R4 are supposed to counteract TRAIL-R1 and TRAIL-R2. Here we have aimed to present briefly the role of TRAIL-mediated cell death in viral and bacterial infections, and propose that while apoptosis may not be the root cause of these diseases, TRAIL-based therapy would mitigate the pathology and improve health in many disease states.
References
Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–5.
Lunemann JD, Waiczies S, Ehrlich S, et al. Death ligand TRAIL induces no apoptosis but inhibits activation of human (auto)antigen-specific T cells. J Immunol. 2002;168:4881–8.
Song K, Chen Y, Goke R, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–104.
Walczak H, Haas TL. Biochemical analysis of the native TRAIL death-inducing signaling complex. Methods Mol Biol. 2008;414:221–39.
Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–14.
Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23.
Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60.
Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–5.
Schneider P, Olson D, Tardivel A, et al. Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Biol Chem. 2003;278:5444–54.
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–8.
Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–82.
Jeremias I, Herr I, Boehler T, Debatin KM. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–52.
Mariani SM, Krammer PH. Surface expression of TRAIL/Apo-2 ligand in activated mouse T and B cells. Eur J Immunol. 1998;28:1492–8.
Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–90.
Bossi F, Bernardi S, Zauli G, Secchiero P, Fabris B. TRAIL modulates the immune system and protects against the development of diabetes. J Immunol Res. 2015;2015:680749.
Zauli G, Sancilio S, Cataldi A, Sabatini N, Bosco D, Di Pietro R. PI-3K/Akt and NF-kappaB/IkappaBalpha pathways are activated in Jurkat T cells in response to TRAIL treatment. J Cell Physiol. 2005;202:900–11.
Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med. 1998;188:2375–80.
Johnsen AC, Haux J, Steinkjer B, et al. Regulation of APO-2 ligand/trail expression in NK cells-involvement in NK cell-mediated cytotoxicity. Cytokine. 1999;11:664–72.
Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–54.
Leverkus M, Walczak H, McLellan A, et al. Maturation of dendritic cells leads to up-regulation of cellular FLICE-inhibitory protein and concomitant down-regulation of death ligand-mediated apoptosis. Blood. 2000;96:2628–31.
Lamhamedi-Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat Immunol. 2003;4:255–60.
Ikeda T, Hirata S, Fukushima S, et al. Dual effects of TRAIL in suppression of autoimmunity: the inhibition of Th1 cells and the promotion of regulatory T cells. J Immunol. 2010;185:5259–67.
Roberts AI, Devadas S, Zhang X, et al. The role of activation-induced cell death in the differentiation of T-helper-cell subsets. Immunol Res. 2003;28:285–93.
Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93.
Diehl GE, Yue HH, Hsieh K, et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–89.
Tanji H, Ohto U, Shibata T, Miyake K, Shimizu T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science. 2013;339:1426–9.
Perry AK, Chen G, Zheng D, Tang H, Cheng G. The host type I interferon response to viral and bacterial infections. Cell Res. 2005;15:407–22.
TenOever BR, Sharma S, Zou W, et al. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J Virol. 2004;78:10636–49.
Krug A, French AR, Barchet W, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19.
Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–7.
Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–20.
Muller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21.
Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–63.
Mordstein M, Neugebauer E, Ditt V, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J Virol. 2010;84:5670–7.
Mordstein M, Kochs G, Dumoutier L, et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151.
Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat Commun. 2014;5:3864.
Durbin JE, Fernandez-Sesma A, Lee CK, et al. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164:4220–8.
Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74:3996–4003.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8.
Doyle S, Vaidya S, O’Connell R, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–63.
Hibma MH. The immune response to papillomavirus during infection persistence and regression. Open Virol J. 2012;6:241–8.
Clarke P, Meintzer SM, Gibson S, et al. Reovirus-induced apoptosis is mediated by TRAIL. J Virol. 2000;74:8135–9.
Lum JJ, Pilon AA, Sanchez-Dardon J, et al. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J Virol. 2001;75:11128–36.
Sato K, Hida S, Takayanagi H, et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-alpha/beta. Eur J Immunol. 2001;31:3138–46.
Brincks EL, Katewa A, Kucaba TA, Griffith TS, Legge KL. CD8 T cells utilize TRAIL to control influenza virus infection. J Immunol. 2008;181:4918–25.
Herold S, Steinmueller M, von Wulffen W, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–77.
Ciesek S, Liermann H, Hadem J, et al. Impaired TRAIL-dependent cytotoxicity of CD1c-positive dendritic cells in chronic hepatitis C virus infection. J Viral Hepat. 2008;15:200–11.
Glassner A, Eisenhardt M, Kramer B, et al. NK cells from HCV-infected patients effectively induce apoptosis of activated primary human hepatic stellate cells in a TRAIL-, FasL- and NKG2D-dependent manner. Lab Invest. 2012;92:967–77.
Jang JY, Kim SJ, Cho EK, et al. TRAIL enhances apoptosis of human hepatocellular carcinoma cells sensitized by hepatitis C virus infection: therapeutic implications. PLoS One. 2014;9:e98171.
Liu FW, Wu DB, Chen EQ, et al. Expression of TRAIL in liver tissue from patients with different outcomes of HBV infection. Clin Res Hepatol Gastroenterol. 2013;37:269–74.
Falschlehner C, Schaefer U, Walczak H. Following TRAIL’s path in the immune system. Immunology. 2009;127:145–54.
Stacey MA, Marsden M, Pham NT, et al. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell Host Microbe. 2014;15:471–83.
Goulding J, Abboud G, Tahiliani V, Desai P, Hutchinson TE, Salek-Ardakani S. CD8 T cells use IFN-gamma to protect against the lethal effects of a respiratory poxvirus infection. J Immunol. 2014;192:5415–25.
Arias J, Valero N, Mosquera J, et al. Increased expression of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology. 2014;452–453:42–51.
Becerra A, Warke RV, Martin K, et al. Gene expression profiling of dengue infected human primary cells identifies secreted mediators in vivo. J Med Virol. 2009;81:1403–11.
Herbeuval JP, Grivel JC, Boasso A, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–31.
Hansjee N, Kaufmann GR, Strub C, Weber R, Battegay M, Erb P. Persistent apoptosis in HIV-1-infected individuals receiving potent antiretroviral therapy is associated with poor recovery of CD4 T lymphocytes. J Acquir Immune Defic Syndr. 2004;36:671–7.
Gougeon ML. To kill or be killed: how HIV exhausts the immune system. Cell Death Differ. 2005;12(Suppl 1):845–54.
Roumier T, Vieira HL, Castedo M, et al. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 2002;9:1212–9.
Park SY, Waheed AA, Zhang ZR, Freed EO, Bonifacino JS. HIV-1 Vpu accessory protein induces caspase-mediated cleavage of IRF3 transcription factor. J Biol Chem. 2014;289:35102–10.
Unwalla H, Chakraborti S, Sood V, Gupta N, Banerjea AC. Potent inhibition of HIV-1 gene expression and TAT-mediated apoptosis in human T cells by novel mono- and multitarget anti-TAT/Rev/Env ribozymes and a general purpose RNA-cleaving DNA-enzyme. Antiviral Res. 2006;72:134–44.
Rasola A, Gramaglia D, Boccaccio C, Comoglio PM. Apoptosis enhancement by the HIV-1 Nef protein. J Immunol. 2001;166:81–8.
Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–9.
Hou WK, Meng LS, Zheng F, et al. Methotrexate ameliorates pristane-induced arthritis by decreasing IFN-gamma and IL-17A expressions. J Zhejiang Univ Sci B. 2011;12:40–6.
Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96.
Alexander DE, Leib DA. Xenophagy in herpes simplex virus replication and pathogenesis. Autophagy. 2008;4:101–3.
Schmid D, Pypaert M, Munz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92.
Suhy DA, Giddings TH Jr, Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J Virol. 2000;74:8953–65.
Wong J, Zhang J, Si X, et al. Autophagosome supports coxsackievirus B3 replication in host cells. J Virol. 2008;82:9143–53.
Yordy B, Iwasaki A. Autophagy in the control and pathogenesis of viral infection. Curr Opin Virol. 2011;1:196–203.
Kotelkin A, Prikhod’ko EA, Cohen JI, Collins PL, Bukreyev A. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J Virol. 2003;77:9156–72.
Ruckdeschel K, Roggenkamp A, Lafont V, Mangeat P, Heesemann J, Rouot B. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun. 1997;65:4813–21.
Wickstrum JR, Bokhari SM, Fischer JL, et al. Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect Immun. 2009;77:4827–36.
Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8:668–74.
Giogha C, Lung TW, Pearson JS, Hartland EL. Inhibition of death receptor signaling by bacterial gut pathogens. Cytokine Growth Factor Rev. 2014;25:235–43.
Luo J, Hu J, Zhang Y, Hu Q, Li S. Hijacking of death receptor signaling by bacterial pathogen effectors. Apoptosis. 2015;20:216–23.
Rich KA, Burkett C, Webster P. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol. 2003;5:455–68.
Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66.
Jones ME, Karlowsky JA, Draghi DC, Thornsberry C, Sahm DF, Nathwani D. Antibiotic susceptibility of bacteria most commonly isolated from bone related infections: the role of cephalosporins in antimicrobial therapy. Int J Antimicrob Agents. 2004;23:240–6.
Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res. 2004;30:291–308.
Henderson B, Nair SP. Hard labour: bacterial infection of the skeleton. Trends Microbiol. 2003;11:570–7.
Alexander EH, Rivera FA, Marriott I, Anguita J, Bost KL, Hudson MC. Staphylococcus aureus—induced tumor necrosis factor—related apoptosis—inducing ligand expression mediates apoptosis and caspase-8 activation in infected osteoblasts. BMC Microbiol. 2003;3:5.
Young AB, Cooley ID, Chauhan VS, Marriott I. Causative agents of osteomyelitis induce death domain-containing TNF-related apoptosis-inducing ligand receptor expression on osteoblasts. Bone. 2011;48:857–63.
Zhong J, Gilbertson B, Cheers C. Apoptosis of CD4+ and CD8+ T cells during experimental infection with Mycobacterium avium is controlled by Fas/FasL and Bcl-2-sensitive pathways, respectively. Immunol Cell Biol. 2003;81:480–6.
Borges M, Rosa GT, Appelberg R. The death-promoting molecule tumour necrosis factor-related apoptosis inducing ligand (TRAIL) is not required for the development of peripheral lymphopenia or granuloma necrosis during infection with virulent Mycobacterium avium. Clin Exp Immunol. 2011;164:407–16.
Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90.
Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–703.
Moss SF, Calam J, Agarwal B, Wang S, Holt PR. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501.
Lin WC, Tsai HF, Liao HJ, et al. Helicobacter pylori sensitizes TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human gastric epithelial cells through regulation of FLIP. Cell Death Dis. 2014;5:e1109.
Zheng SJ, Jiang J, Shen H, Chen YH. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J Immunol. 2004;173:5652–8.
Gurung P, Rai D, Condotta SA, Babcock JC, Badovinac VP, Griffith TS. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J Immunol. 2011;187:2148–54.
Campbell LA, Patton DL, Moore DE, Cappuccio AL, Mueller BA, Wang SP. Detection of Chlamydia trachomatis deoxyribonucleic acid in women with tubal infertility. Fertil Steril. 1993;59:45–50.
Al-Kuhlani M, Rothchild J, Pal S, et al. TRAIL-R1 is a negative regulator of pro-inflammatory responses and modulates long-term sequelae resulting from Chlamydia trachomatis infections in humans. PLoS One. 2014;9:e93939.
Qiu H, Fan Y, Joyee AG, et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J Immunol. 2008;181:2092–102.
Steinwede K, Henken S, Bohling J, et al. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med. 2012;209:1937–52.
Hoffmann O, Priller J, Prozorovski T, et al. TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest. 2007;117:2004–13.
Beyer K, Poetschke C, Partecke LI, et al. TRAIL induces neutrophil apoptosis and dampens sepsis-induced organ injury in murine colon ascendens stent peritonitis. PLoS One. 2014;9:e97451.
Leverkus M, Neumann M, Mengling T, et al. Regulation of tumor necrosis factor-related apoptosis-inducing ligand sensitivity in primary and transformed human keratinocytes. Cancer Res. 2000;60:553–9.
Jansen BJ, van Ruissen F, Cerneus S, et al. Tumor necrosis factor related apoptosis inducing ligand triggers apoptosis in dividing but not in differentiating human epidermal keratinocytes. J Invest Dermatol. 2003;121:1433–9.
Sedger LM, Shows DM, Blanton RA, et al. IFN-gamma mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J Immunol. 1999;163:920–6.
Neu B, Rad R, Reindl W, et al. Expression of tumor necrosis factor- alpha -related apoptosis-inducing ligand and its proapoptotic receptors is down-regulated during gastric infection with virulent cagA+/vacAs1+ Helicobacter pylori strains. J Infect Dis. 2005;191:571–8.
Hao C, Song JH, Hsi B, et al. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res. 2004;64:8502–6.
Kelley RF, Totpal K, Lindstrom SH, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–12.
Camidge DR, Herbst RS, Gordon MS, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–63.
Sharma S, de Vries EG, Infante JR, et al. Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Invest New Drugs. 2014;32:135–44.
Wakelee HA, Patnaik A, Sikic BI, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21:376–81.
Wang H, Davis JS, Wu X. Immunoglobulin Fc domain fusion to TRAIL significantly prolongs its plasma half-life and enhances its antitumor activity. Mol Cancer Ther. 2014;13:643–50.
Acknowledgments
This work was supported by a Grant B01/6 from the National Science Fund, Ministry of Education and Science, Bulgaria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Mauro Teixeira.
Rights and permissions
About this article
Cite this article
Gyurkovska, V., Ivanovska, N. Distinct roles of TNF-related apoptosis-inducing ligand (TRAIL) in viral and bacterial infections: from pathogenesis to pathogen clearance. Inflamm. Res. 65, 427–437 (2016). https://doi.org/10.1007/s00011-016-0934-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0934-1