Abstract
Objective
The activation of nuclear factor (NF)-κB by cytokines under hyperglycaemic conditions is a potential mechanism for complications in diabetes. We investigated whether small ubiquitin-like modifier 4 (SUMO4) regulates renal NF-κB signalling in diabetic rats.
Methods
Histological changes in kidney were analysed in diabetic GK rats. The expressions of tumour necrosis factor (TNF)-α, NF-κB (p65), IκBα and SUMO4 in renal tissues were examined by immunohistochemistry and Western blotting. Primary cultured glomerular endothelial cells from rats were stimulated by TNF-α or interleukin (IL)-2.
Results
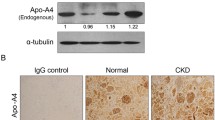
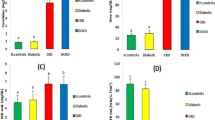
The renal expression of TNF-α, NF-κB (p65), IκBα and SUMO4 was significantly higher in diabetic GK rats than in control rats. In control rats, no nuclear translocation was observed for IκBα or NF-κB (p65). However, in diabetic GK rats, translocation of NF-κB (p65) and IκBα into the nucleus was observed, and the expression of SUMO4 and IκBα was up-regulated in the glomerular endothelial cells. SUMO4 was localised in both the cytoplasm and nucleus, while IκBα was predominantly located in the nucleus after stimulation with TNF-α. In contrast, SUMO4 was localised in the nucleus, and increased cytoplasm SUMO4 localisation was found after stimulation with IL-2.
Conclusions
SUMO4 plays a role in regulating NF-κB signalling in glomerular cells. Cytokines have a unique effect in regulating the sumoylation of NF-κB.





Similar content being viewed by others
References
Nguyen DV, Shaw LC, Grant MB. Inflammation in the pathogenesis of microvascular complications in diabetes. Front Endocrinol. 2012;3:170. doi:10.3389/fendo.2012.00170.
Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (London). 2009;23(7):1496–508. doi:10.1038/eye.2009.108.
Patel S, Santani D. Role of NF-kappa B in the pathogenesis of diabetes and its associated complications. Pharmacol Rep. 2009;61(4):595–603.
Morita M, Yano S, Yamaguchi T, Sugimoto T. Advanced glycation end products-induced reactive oxygen species generation is partly through NF-kappa B activation in human aortic endothelial cells. J Diabetes Complications. 2013;27(1):11–5. doi:10.1016/j.jdiacomp.2012.07.006.
Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12(1):85–98. doi:10.1006/smim.2000.0210.
de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25(5):904–14. doi:10.1161/01.ATV.0000160340.72641.87.
Mabb AM, Miyamoto S. SUMO and NF-kappaB ties. Cell Mol Life Sci. 2007;64(15):1979–96. doi:10.1007/s00018-007-7005-2.
Lomeli H, Vazquez M. Emerging roles of the SUMO pathway in development. Cell Mol Life Sci. 2011;68(24):4045–64. doi:10.1007/s00018-011-0792-5.
Guo D, Li M, Zhang Y, Yang P, Eckenrode S, Hopkins D, et al. A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat Genet. 2004;36(8):837–41. doi:10.1038/ng1391.
Wang CY, Yang P, Li M, Gong F. Characterization of a negative feedback network between SUMO4 expression and NFkappaB transcriptional activity. Biochem Biophys Res Commun. 2009;381(4):477–81. doi:10.1016/j.bbrc.2009.02.060.
Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259(5103):1912–5.
Xu X, Wang P, Zhao Z, Cao T, He H, Luo Z, et al. Activation of transient receptor potential vanilloid 1 by dietary capsaicin delays the onset of stroke in stroke-prone spontaneously hypertensive rats. Stroke J Cereb Circ. 2011;42(11):3245–51. doi:10.1161/STROKEAHA.111.618306.
Ruster C, Franke S, Wenzel U, Schmidthaupt R, Fraune C, Krebs C, et al. Podocytes of AT2 receptor knockout mice are protected from angiotensin II-mediated RAGE induction. Am J Nephrol. 2011;34(4):309–17. doi:10.1159/000329321.
Nagao T, Matsumura M, Mabuchi A, Ishida-Okawara A, Koshio O, Nakayama T, et al. Up-regulation of adhesion molecule expression in glomerular endothelial cells by anti-myeloperoxidase antibody. Nephrol Dial Transplant: Off Publ Eur Dial Transpl Assoc Eur Renal Assoc. 2007;22(1):77–87. doi:10.1093/ndt/gfl555.
Chen X, Yang D, Ma S, He H, Luo Z, Feng X, et al. Increased rhythmicity in hypertensive arterial smooth muscle is linked to transient receptor potential canonical channels. J Cell Mol Med. 2010;14(10):2483–94. doi:10.1111/j.1582-4934.2009.00890.x.
Liu D, Yang D, He H, Chen X, Cao T, Feng X, et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53(1):70–6. doi:10.1161/HYPERTENSIONAHA.108.116947.
Ciszewicz M, Polubinska A, Antoniewicz A, Suminska-Jasinska K, Breborowicz A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Transl Res: J Lab Clin Med. 2009;153(3):118–23. doi:10.1016/j.trsl.2008.12.007.
Chen L, Zhang J, Zhang Y, Wang Y, Wang B. Improvement of inflammatory responses associated with NF-kappa B pathway in kidneys from diabetic rats. Inflamm Res: Off J Eur Histamine Res Soc. 2008;57(5):199–204. doi:10.1007/s00011-006-6190-z.
Karon J, Polubinska A, Antoniewicz AA, Suminska-Jasinska K, Breborowicz A. Anti-inflammatory effect of sulodexide during acute peritonitis in rats. Blood Purif. 2007;25(5–6):510–4. doi:10.1159/000113011.
Acknowledgments
We thank Lijuan Wang (Chongqing Institute of Hypertension, China) for technical assistance. This research was supported by grants from the National Basic Research Program of China (2012CB517806) and National Natural Science Foundation of China (81130006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Liwu Li.
Sijiao Chen and Tao Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, S., Yang, T., Liu, F. et al. Inflammatory factor-specific sumoylation regulates NF-κB signalling in glomerular cells from diabetic rats. Inflamm. Res. 63, 23–31 (2014). https://doi.org/10.1007/s00011-013-0675-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0675-3