Abstract
Objective and design
Patients with ulcerative colitis have increased risk of colorectal carcinoma, but little is known about how peritoneal macrophages are involved in ulcerative colitis-associated carcinogenesis. We investigated the alteration of peritoneal macrophages and M1/M2 subpopulations during ulcerative colitis-associated carcinogenesis.
Materials and methods
Expression and functional changes in peritoneal macrophages and M1/M2 subpopulations were investigated by histopathology, flow cytometry, immunofluorescence, cytokines expression by ELISA and QRT-PCR in an azoxymethane (AOM)- and dextran sodium sulfate (DSS)-induced chemical colitis-associated carcinoma mouse model using male Crj:CD-1 (ICR) mice.
Results
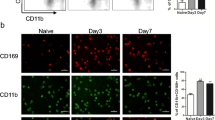
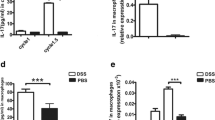
Striking evidence observed in histopathology, flow cytometry, cytokine detection, and gene expression analysis all revealed that inflammation-associated cytokines (IL-1β, IL-10, IL-12, IL-6, TNF-α) and migration/invasion-associated factors (G-CSF, GM-CSF, CXCR4, VEGF, TGF-β, ICAM-1) induced by peritoneal M2 macrophages increased significantly during the progression from inflammatory hyperplasia to carcinoma and metastasis. Similar functional changes occurred during peritoneal metastasis in M1 macrophages without changed polarization.
Conclusions
These results suggested that peritoneal M2 macrophages played a critical role in ulcerative colitis-associated carcinogenesis, including unbalanced pro-inflammatory and anti-inflammatory axis and enhanced expression of migration/invasion-associated factors. Furthermore, functional changes of M1 macrophages occurred without changed polarization during carcinogenesis and metastasis.






Similar content being viewed by others
References
Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–82.
Rod R. Crohn’s disease and ulcerative colitis: morbidity and mortality. Health Rep. 1990;2:343–59.
Loftus EV. J Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17.
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.
Hanauer S. Inflammatory bowel disease: epidemiology, pathogenesis and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–9.
Lakatos L, Mester G, Erdelyi Z, et al. Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis. 2006;12:205–11.
Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–47.
Jemal A, Bray F, Center MM, et al. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90.
Gong W, Lv N, Wang B, et al. Risk of ulcerative colitis-associated colorectal cancer in China: a multi-center retrospective study. Dig Dis Sci. 2012;57:503–7.
Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14:2662–9.
Karin M. Nuclear factor-kappa B in cancer development and progression. Nature. 2006;441:431–6.
Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6.
Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-Mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumor genesis. Cancer Cell. 2009;15:91–102.
Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13.
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57.
Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102.
Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–89.
Bar-On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin Immunol. 2011;23:58–64.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61.
Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;1:453–61.
Sica A, Schioppa T, Mantovani A, et al. Tumor associated macrophages are a distinct M2 polarised population promoting tumor progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27.
Bailey C, Negus R, Morris A, et al. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metast. 2007;24:121–30.
Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73.
Green CE, Liu T, Montel V, et al. Chemoattractant signaling between tumor cells and macrophages regulated cancer cell migration, metastasis and neovascularization. PLoS ONE. 2009;4:e6713.
Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–6.
Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49.
Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–9.
Lai CS, Tsai ML, Cheng AC, et al. Chemoprevention of colonic tumorigenesis by dietary hydroxylated polymethoxyflavones in azoxymethane-treated mice. Mol Nutr Food Res. 2011;55:278–90.
Tang A, Li N, Li X, et al. Dynamic activation of the key pathways: linking colitis to colorectal cancer in a mouse mode. Carcinogenesis. 2012;33:1375–83.
Hanahan D, Weinberg RA. Hallmarks of Cancer: the next generation. Cell. 2011;144:646–74.
Lutgens MW, Vleggaar FP, Schipper ME, et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246–51.
Forssell J, Oberg A, Henriksson ML, et al. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–9.
Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6.
Mantovani A. Inflammation and cancer: the macrophage connection. Medicina. 2007;67:32–4.
Erreni M, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron. 2011;4:141–54.
Moriya M, Harada T, Shirasu Y. Inhibition of carcinogenicities of 1,2-dimethylhydrazine and azoxymethane by pyrazole. Cancer Lett. 1982;17:147–52.
Sugie S, Mori Y, Okumura A, et al. Promoting and synergistic effects of chrysazin on 1,2-dimethylhydrazine-induced carcinogenesis in male ICR/CD-1 mice. Carcinogenesis. 1994;15:1175–9.
Nardin A, Abastado JP. Macrophages and cancer. Front Biosci. 2008;13:3494–505.
Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15.
Imtiyaz HZ, Williams EP, Hickey MM, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–714.
Low-Marchelli JM, Ardi VC, Vizcarra EA, et al. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–71.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81172300, 81272975, 81272736), Key Project of Hunan Provincial Natural Science Foundation (12JJ2044), Project of Hunan Provincial Development and Reform Commission, Hunan Provincial Innovation Foundation for Postgraduate (CX2011B073), and Mittal Student Innovation Project (11MX25). We are grateful to the members of our laboratory, Professor Songqing Fan and Lei Shi (The Second Xiangya Hospital), and technical support of Beckman Coulter and Medjaden Bioscience Limited.
Conflict of interest
The authors have no financial or other potential conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Michael J. Parnham.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, W., Li, X., Zheng, D. et al. Dynamic changes of peritoneal macrophages and subpopulations during ulcerative colitis to metastasis of colorectal carcinoma in a mouse model. Inflamm. Res. 62, 669–680 (2013). https://doi.org/10.1007/s00011-013-0619-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0619-y