Abstract
Objective
The endotoxin tolerance phenotype is characterized with decreased inflammation and increased phagocytosis. We hypothesized that endotoxin tolerance would provide protective effects on experimental sepsis with multiple organ injuries induced by cecal ligation and puncture (CLP).
Methods
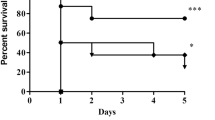
Endotoxin tolerance was induced in male Sprague–Dawley rats with daily intraperitoneal injection of either 0.6 mg/kg of lipopolysaccharide (LPS) or vehicle for four consecutive days before subsequent CLP. Biochemical parameters, histological changes, inflammatory cytokine production, and lung tissue nuclear factor-κB (NF-κB) activation were assessed post-CLP. In a separate experiment, survival rate was monitored for 7 days after CLP.
Results
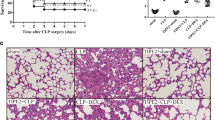
In vehicle-treated animals, CLP caused multiple organ injuries confirmed by the biochemical variables and histological examination. This was accompanied by an early activation of NF-κB in the lung and a substantial increase in plasma levels of tumor necrosis factor-α, interleukin-6, and interleukin-10. In contrast, pretreatment with LPS not only alleviated the development of multiple organ injuries after CLP, but also decreased sepsis-induced activation of pulmonary NF-κB and reduced plasma cytokines production. In addition, LPS pretreatment improved the survival in rats subjected to CLP.
Conclusions
The beneficial effects of endotoxin tolerance indicate the potential of immunomodulatory strategies in the management of severe sepsis.





Similar content being viewed by others
References
Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Eng J Med. 2003;348:1546–54.
Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–83.
Wheeler AP. Recent developments in the diagnosis and management of severe sepsis. Chest. 2007;132:1967–76.
Vincent JL, Abraham E. The last 100 years in sepsis. Am J Resp Crit Care Med. 2006;173:256–63.
Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. Novel therapies for sepsis: a review. J Trauma. 2005;58:867–74.
Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78.
Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44.
Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009;25:677–702.
Cohen J. Non-antibiotic strategies for sepsis. Clin Microbiol Infect. 2009;15:302–7.
Schefold JC, Hasper D, Volk HD, Reinke P. Sepsis: time has come to focus on the later stages. Med Hypotheses. 2008;71:203–8.
West MA, Heagy W. Endotoxin tolerance: a review. Crit Care Med. 2002;30(1 supp):S64–73.
Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84.
Beeson PB. Development of tolerance to typhoid bacterial pyrogen and its abolition by reticuloendothelial blockade. Proc Soc Exp Biol Med. 1946;61:248–50.
Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–87.
Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon JM. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68:3748–53.
Lehner MD, Ittner J, Bundschuh DS, van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69:463–71.
Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, et al. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008;30:267–73.
Poli-de-Figueiredo LF, Garrido AG, Nakagawa N, Sannomiya P. Experimental models of sepsis and their clinical relevance. Shock. 2008;30(Suppl 1):53–9.
Sheehan M, Wong HR, Hake PW, Zingarelli B. Parthenolide improves systemic hemodynamics and decreases tissue leukosequestration in rats with polymicrobial sepsis. Crit Care Med. 2003;31:2263–70.
Qu J, Zhang J, Pan J, He L, Ou Z, Zhang X, et al. Endotoxin tolerance inhibits lipopolysaccharide-initiated acute pulmonary inflammation and lung injury in rats by the mechanism of nuclear factor-kappaB. Scand J Immunol. 2003;58:613–9.
Sener G, Toklu H, Kapucu C, Ercan F, Erkanli G, Kaçmaz A, et al. Melatonin protects against oxidative organ injury in a rat model of sepsis. Surg Today. 2005;35:52–9.
Wheeler DS, Lahni PM, Hake PW, Denenberg AG, Wong HR, Snead C, et al. The green tea polyphenol epigallocatechin-3-gallate improves systemic hemodynamics and survival in rodent models of polymicrobial sepsis. Shock. 2007;28:353–9.
Singleton KD, Beckey VE, Wischmeyer PE. Glutamine prevents activation of NF-kappaB and stress kinase pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock. 2005;24:583–9.
Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–74.
Varma TK, Durham M, Murphey ED, Cui W, Huang Z, Lin CY, et al. Endotoxin priming improves clearance of Pseudomonas aeruginosa in wild-type and interleukin-10 knockout mice. Infect Immun. 2005;73:7340–7.
Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233–41.
Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–8.
Zhang J, Qu JM, He LX. IL-12 suppression, enhanced endocytosis and up-regulation of MHC-II and CD80 in dendritic cells during experimental endotoxin tolerance. Acta Pharmacol Sin. 2009;30:582–8.
Acknowledgments
The authors wish to thank Dr. Hong-liang Zong for his excellent technical assistance and Dr. Vincent VanBuren (Texas A&M University, Health Science Center) for his careful review of the article. This work was supported by Grants from the State Natural Sciences Foundation project (No. 30770930), the Program of Shanghai Subject Chief Scientist (No. 07XD14012), Shanghai Leading Talent Projects (2010No.036 to JMQ), and National Basic Research Program of China (973 Program) (No. 2007CB513004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Dong-Wei Shi and Jing Zhang contributed equally to this article.
Rights and permissions
About this article
Cite this article
Shi, DW., Zhang, J., Jiang, HN. et al. LPS pretreatment ameliorates multiple organ injuries and improves survival in a murine model of polymicrobial sepsis. Inflamm. Res. 60, 841–849 (2011). https://doi.org/10.1007/s00011-011-0342-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0342-5