Abstract
Objective
To observe the therapeutic effect of RelB-silenced dendritic cells (DCs) in experimental autoimmune myasthenia gravis (EAMG), and further to investigate the mechanism of this immune system therapeutic.
Methods
(1) RelB-silenced DCs and control DCs were prepared and the supernatants were collected for IL-12p70, IL-6, and IL-23 measurement by ELISA. (2) RelB-silenced DCs and control DCs were co-cultured with AChR-specific T cells, and the supernatant was collected to observe the IL-17, IFN-γ, IL-4 production. (3) EAMG mice with clinical scores of 1 to 2 were collected and enrolled randomly into the RelB-silenced DC group or the control DC group. RelB-silenced DCs or an equal amount of control DCs were injected intravenously on days 0, 7, and 14 after enrollment. Clinical scores were evaluated every other day. Twenty days after allotment, serum from individual mice was collected to detect serum concentrations of anti-mouse AChR IgG, IgG1, IgG2b, and IgG2c. The splenocytes were isolated for analysis of lymphocyte proliferative responses, cytokine (IL-17, IFN-γ, IL-4) production, and protein levels of RORγt, T-bet, GATA-3, and FoxP3 (the special transcription factors of Th17, Th1, Th2, and Treg, respectively).
Results
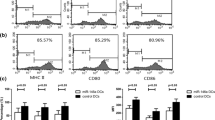
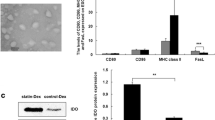
(1) RelB-silenced DCs produced significantly reduced amounts of IL-12p70, IL-6, and IL-23, as compared with control DCs. (2) RelB-silenced DCs spurred on the CD4+ T cells from Th1/Th17 to the Th2 cell subset in the co-culture system. (3) Treatment with RelB-silenced DCs effectively reduced myasthenic symptoms and levels of serum anti-acetylcholine receptor autoantibody in mice with ongoing EAMG. Th17-related markers (RORγt, IL-17) and Th1-related markers (T-bet, IFN-γ) were downregulated, whereas Th2 markers (IL-4 and GATA3) and Treg marker (FoxP3) were upregulated.
Conclusions
RelB-silenced DCs were able to create a particular cytokine environment that was absent of inflammatory cytokines. RelB-silenced DCs provide a practical means to normalize the differentiation of the four T-cell subsets (Th17, Th1, Th2, and Treg) in vivo, and thus possess therapeutic potential in Th1/Th17-dominant autoimmune disorders such as myasthenia gravis.





Similar content being viewed by others
References
Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122–8.
Berman PW, Patrick J. Linkage between the frequency of muscular weakness and loci that regulate immune responsiveness in murine experimental myasthenia gravis. J Exp Med. 1980;151:204–23.
Hughes BW, Moro De Casillas ML, Kaminski HJ. Pathophysiology of myasthenia gravis. Semin Neurol. 2004;24:21–30.
Elson CJ, Barker RN. Helper T cells in antibody-mediated, organ-specific autoimmunity. Curr Opin Immunol. 2000;12:664–9.
Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–44.
Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73.
Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–41.
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–33.
Wang W, Milani M, Ostlie N, Okita D, Agarwal RK, Caspi RR, et al. C57BL/6 mice genetically deficient in IL-12/IL-23 and IFN-gamma are susceptible to experimental autoimmune myasthenia gravis, suggesting a pathogenic role of non-Th1 cells. J Immunol. 2007;178:7072–80.
Bai Y, Liu R, Huang D, La Cava A, Tang YY, Iwakura Y, et al. CCL2 recruitment of IL-6-producing CD11b+ monocytes to the draining lymph nodes during the initiation of Th17-dependent B cell-mediated autoimmunity. Eur J Immunol. 2008;38:1877–88.
Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Ann NY Acad Sci. 2007;1103:179–91.
Zhang Y, Yang H, Xiao B, Wu M, Zhou W, Li J, et al. Dendritic cells transduced with lentiviral-mediated RelB-specific ShRNAs inhibit the development of experimental autoimmune myasthenia gravis. Mol Immunol. 2009;46:657–67.
Yang H, Goluszko E, David C, Okita DK, Conti-Fine B, Chan TS, et al. Mapping myasthenia gravis-associated T cell epitopes on human acetylcholine receptors in HLA transgenic mice. J Clin Invest. 2002;109(8):1111–20.
Wu B, Goluszko E, Christadoss P. Experimental autoimmune myasthenia gravis in the mouse. Curr Protoc Immunol. 2001; chapter 15: unit 15.8.
Yang H, Kala M, Scott BG, Goluszko E, Chapman HA, Christadoss P. Cathepsin S is required for murine autoimmune myasthenia gravis pathogensis. J Immunol. 2005;174:1729–37.
Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–41.
Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907–14.
Lu L, Li W, Zhong C, Qian S, Fung JJ, Thomson AW, et al. Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression, are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb. Transplantation. 1999;68(6):747–57.
Nouri-Shirazi M, Guinet E. Direct and indirect cross-tolerance of alloreactive T cells by dendritic cells retained in the immature stage. Transplantation. 2002;74:1035–44.
Buonocore S, Flamand V, Goldman M, Braun MY. Bone marrow derived immature dendritic cells prime in vivo alloreactive T cells for interleukin-4-dependent rejection of major histocompatibility complex class II antigen-disparate cardiac allograft. Transplantation. 2003;75(3):407–13.
Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168(1):171–8.
Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436(7054):1181–5.
Martin E, O’Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003;18(1):155–67.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–8.
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–11.
Hannon GJ. RNA interference. Nature. 2002;418(6894):244–51.
Ageichik A, Collins MK, Dewannieux M. Lentivector targeting to dendritic cells. Mol Ther. 2008;16(6):1008–9.
Jiang HR, Muckersie E, Robertson M, Forrester JV. Antigen-specific inhibition of experimental autoimmune uveoretinitis by bone marrow-derived immature dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:1598–607.
Waibler Z, Kalinke U, Will J, Juan MH, Pfeilschifter JM, Radeke HH. TLR-ligand stimulated interleukin-23 subunit expression and assembly is regulated differentially in murine plasmacytoid and myeloid dendritic cells. Mol Immunol. 2007;44:1483–9.
Milani M, Ostlie N, Wu H, Wang W, Conti-Fine BM. CD4+ T and B cells cooperate in the immunoregulation of experimental autoimmune myasthenia gravis. J Neuroimmunol. 2006;179(1–2):152–62.
Saoudi A, Bernard I, Hoedemaekers A, Cautain B, Martinez K, Druet P, et al. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1- or Th2-type immune response in rats. J Immunol. 1999;162(12):7189–97.
Balasa B, Deng C, Lee J, Bradley LM, Dalton DK, Christadoss P, et al. Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med. 1997;186(3):385–91.
Zhang GX, Xiao BG, Bai XF, van der Meide PH, Orn A, Link H. Mice with IFN-gamma receptor deficiency are less susceptible to experimental autoimmune myasthenia gravis. J Immunol. 1999;162(7):3775–81.
Balasa B, Deng C, Lee J, Christadoss P, Sarvetnick N. The Th2 cytokine IL-4 is not required for the progression of antibody-dependent autoimmune myasthenia gravis. J Immunol. 1998;161(6):2856–62.
Karachunski PI, Ostlie NS, Okita DK, Conti-Fine BM. Interleukin-4 deficiency facilitates development of experimental myasthenia gravis and precludes its prevention by nasal administration of CD4+ epitope sequences of the acetylcholine receptor. J Neuroimmunol. 1999;95(1–2):73–84.
Milani M, Ostlie N, Wang W, Conti-Fine BM. T cells and cytokines in the pathogenesis of acquired myasthenia gravis. Ann NY Acad Sci. 2003;998:284–307.
Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis by granulocyte–macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177(8):5296–306.
Zhang GX, Yu S, Gran B, Li J, Siglienti I, Chen X, et al. Role of IL-12 receptor beta 1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. J Immunol. 2003;171:4485–92.
Liu R, Campagnolo D, Vollmer T, Shi FD. Transcriptional factor T-bet determines the susceptibility to experimental myasthenia gravis. Clin Immunol. 2008;127(Suppl 1):S48.
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8.
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–89.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4.
Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9041–6.
Notley CA, Inglis JJ, Alzabin S, McCann FE, McNamee KE, Williams RO. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med. 2008;205(11):2491–7.
Balandina A, Lécart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–41.
Sun Y, Qiao J, Lu CZ, Zhao CB, Zhu XM, Xiao BG. Increase of circulating CD4+CD25+ T cells in myasthenia gravis patients with stability and thymectomy. Clin Immunol. 2004;112:284–9.
Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24(6):677–88.
Yang J, Bernier SM, Ichim TE, Li M, Xia X, Zhou D, et al. LF15–0195 generates tolerogenic dendritic cells by suppression of NF-κB signaling through inhibition of IKK activity. J Leukoc Biol. 2003;74(3):438–47.
Li M, Zhang X, Zheng X, Lian D, Zhang ZX, Ge W, et al. Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J Immunol. 2007;178:5480–7.
Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–40.
Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19(6):400–8.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30870857) and Hunan Natural Science Foundation (09JJ3079).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: G. Wallace.
H. Yang and Y. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, H., Zhang, Y., Wu, M. et al. Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dentritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm. Res. 59, 197–205 (2010). https://doi.org/10.1007/s00011-009-0087-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-009-0087-6