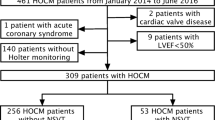
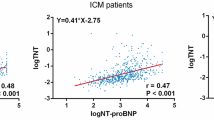
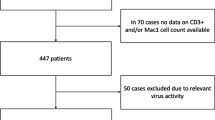
Abstract
Myocyte necrosis has been considered to play a fundamental role in the pathophysiology of congestive heart failure (CHF), which has usually evolved as a consequence of depletion of compensatory mechanisms and contractile reserve of myocardium. Elevated levels of creatine kinase MB (CK-MB) and troponin I (Tn-I) have been regarded as biochemical markers of myocyte necrosis. This study was planned to investigate the specificity and sensitivity of Tn-I and CK-MB in CHF and to examine the correlation of these markers with disease severity. A total of 104 patients (38 female, 66 male; mean age, 66 y [range, 36–89]) with symptoms and signs of heart failure on admission and with a reduced left ventricular ejection fraction (EF; by transthoracic echocardiography) were labeled “the patient group”, and 58 patients (40 female,18 male; mean age, 61 y [range, 34–77]) with no signs or symptoms of CHF and with a normal EF detected by transthoracic echocardiography were included in the study as “the control group.” Left ventricular EFs, end-diastolic diameters, and end-systolic diameters of patients in both groups were measured. Blood samples were drawn from all patients in both groups on admission, so that levels of CK-MB and Tn-I could be measured. All patients in both groups also underwent coronary angiography. Conditions leading to elevation of CK-MB or Tn-I were considered exclusion criteria. The 2 groups failed to show any significant differences in terms of mean age and the presence of coronary artery disease, hypertension, or diabetes mellitus (P > .05). Mean EF in the patient group was lower than that in the control group (P < .05). Mean CK-MB and Tn-I in the patient group were significantly higher than in the control group (P < .05). In the patient group, hypertensive patients were found to have significantly higher mean values of CK-MB than were seen in normotensive patients in the same group (P < .05). In the patient group, 52 cases were considered to be class I-II (New York Heart Association [NYHA]) (group 1), and 52 were considered to be class III-IV (group 2). Group 1, group 2, and the control group did not differ significantly from one another with regard to the presence of coronary artery disease, hypertension, and diabetes mellitus (P > .05).The mean EF in group 2 was significantly lower than that in group 1 and in the control group (P < .05); the mean EF in group 1 was significantly lower than that in the control group (P < .05). Group 1 values did not differ significantly from those of group 2 or the control group in terms of enzymatic markers (P > .05), but group 2 had significantly higher mean values of CK-MB and Tn-I than were noted in the control group (P < .05). The uphill course of CK-MB and Tn-I values from the control group to group 2 (NYHA class III-IV) was statistically significant (P< .05). Serum concentrations of CK-MB and Tn-I may become elevated in severely symptomatic patients with CHF (particularly NYHA class III-IV), demonstrating a relationship between clinical severity of the disease and elevation of myocardial enzymes (CK-MB and Tn-I).
Similar content being viewed by others
References
American Heart Association.Heart Disease and Stroke Statistics-2005 Update. Dallas, Tex: AHA; 2005.
Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J. 1999; 137: 352–360.
Haider KH, Stimson WH. Cardiac troponin-I: a biochemical marker for cardiac cell necrosis.Dis Markers. 1993; 11: 205–215.
Bodor GS, Porterfield D, Voss EM. Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal muscle tissue.Clin Chem. 1995; 41: 1710–1715.
Missov E, Mair J. A novel biochemical approach to congestive heart failure: cardiac troponin T.Am Heart J. 1999; 138: 95–99.
Katz AM.Heart Failure. Philadelphia, Pa: Lippincott Williams and Wilkins; 2000.
Colucci WS. Molecular and cellular mechanisms of myocardial failure.Am J Cardiol. 1997; 80: 15L-25L.
Maytin M, Colucci WS. Molecular and cellular mechanisms of myocardial remodeling.J Nucl Cardiol. 2002; 9: 319–327.
Thaik CM, Calderone A, Takahashi N, Colucci WS. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes.J Clin Invest. 1995; 96: 1093–1099.
Ishii J, Cui W, Kitagawa F, et al. Prognostic value of combination of cardiac troponin T and B-type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem. 2003; 49: 2020–2026.
Goto T, Takase H, Toriyama T, et al. Circulating concentrations of cardiac proteins indicate the severity of congestive heart failure.Heart. 2003; 89: 1303–1307.
Katus HA, Remppis A, Neumann FJ, et al. Diagnostic efficacy of troponin T measurements in acute myocardial infarction.Circulation. 1991; 83: 902–912.
Sato Y, Kita T, Takatsu Y, Kimura T. Biochemical markers of myocyte injury in heart failure.Heart. 2004; 90; 1110–1113.
Sohmiya K, Tanaka T, Tsuji R, et al. Plasma and urinary heart-type cytoplasmic fatty acid-binding protein in coronary occlusion and reperfusion induced myocardial injury model.JMol Cell Cardiol. 1993; 25: 1413–1426.
Del CarloCH, O’Connor CM. Cardiac troponins in congestive heart failure.Am Heart J. 1999; 138: 646–653.
Roongsritong C, Warraich I, Bradley C. Common causes of troponin elevations in the absence of acute myocardial infarction: incidence and clinical significance.Chest. 2004; 125: 1877–1884.
Hein S, Sheffold T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium.J Thorac Cardiovasc Surg. 1995; 110: 89–98.
Katz AM. Cell death in the failing heart: role of an unnatural growth response to overload.Clin Cardiol. 1995; 18: 36–44.
Horwich TB, Patel J, MacLellan WR, Fonarow GC. Cardiac troponin I is associated with impaired hemodynamics, progressive left ventricular dysfunction, and increased mortality rates in advanced heart failure.Circulation. 2003; 108: 833–838.
Schaper J, Elsasser A, Kostin S. The role of cell death in heart failure.Circ Res. 1999; 85: 867–869.
Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure.Circulation. 1997; 96: 2953–2958.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yilmaz, A., Yalta, K., Turgut, O.O. et al. Clinical importance of elevated CK-MB and troponin I levels in congestive heart failure. Adv Therapy 23, 1060–1067 (2006). https://doi.org/10.1007/BF02850226
Issue Date:
DOI: https://doi.org/10.1007/BF02850226