Summary
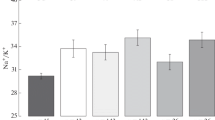
The secretory tubule epithelium of the avian nasal salt gland has been thought to secrete a hyperosmotic fluid containing high concentrations of Naα and Cl−. It is shown by X-ray micro-analysis that the average composition of the luminal fluid of secretory tubules in duckling salt glands is Na 80 mmol l−1, Cl. 100 mmol l−1, K 40 mmol l−1, Mg 11 mmol l−1, Ca 6 mmol l−1. It is assumed that the anion deficit is accounted for by HCO −3 . This fluid is approximately isosmotic or slightly hyposmotic to blood. There were no significant differences between the composition in different regions of the tubule nor between active and inactive glands. The fluid becomes increasingly hyperosmotic in the duct system, most concentration apparently occurring in the main ducts running from gland to external nares. Modification of the primary hyposmotic or isosmotic secretion therefore appears to occur in the duct system.
There were few differences in intracellular composition between the different cell types in the secretory tubule or between active and inactive glands. The average intracellular composition was Na 47 mmol l−1, Cl 54 mmol l−1, K 113 mmol l−1, Mg 14 mmol kg−1, Ca 8 mmol kg−1 wet weight. The high Cl− concentration is suggestive of active Cl− transport. It is suggested that Kα transport into the cells may be a rate limiting process in secretion. It can be calculated that much of the Kα arriving at the gland in the blood must be extracted at high secretion rates. If modification of the primary secretion rccurs in the main duct by water reabsorption, thereby implying a substantially higher secretion rate of primary fluid, then it can be calculated that virtually all of the Kα arriving in the blood may be extracted by the gland.
Similar content being viewed by others
References
Ancey M, Bastenaire F, Tixier R (1977) The statistical control and optimization of X-ray intensity measurements. J Phys D: Appl Phys. 10:817–820
Andrews SB, Mazurkiewicz JE, Kirk GR (1983) The distribution of intracellular ions in the avian salt gland. J Cell Biol 96:1389–1399
Andrews HP, Snee RD, Sarner MH (1980) Graphical display of means. Am Statistician 34:195–199
Baerentsen H, Giraldez F, Zeuthen T (1983) Influx mechanisms for Naα and Cl− across the brush border membrane of leaky epithelia: a model and microelectrode study. J Membr Biol 75:205–218
Beck F, Bauer R, Bauer U, Mason J, Dorge A, Rick R, Thurau K (1980) Electron microprobe analysis of intracellular elements in the rat kidney. Kidney Internat 17:756–763
Bonting SL (1970) Sodium-potassium activated adenosinetriphosphatase and cation transport. In: Bittar EE (ed) Membranes and Ion Transport. Wiley-Interscience, London, pp 257–364
Brown JD, Robinson WH (1979) Quantitative analysis by ϕ (ρζ) curves. In: Newbury DE (ed) Microbeam Analysis. San Francisco Press, San Francisco, pp 238–240
Brown JD, von Rosenstiel AP, Krisch T (1979) Quantitative carbon analysis from ϕ (ρζ) curves. In Newbury DE (ed) Microbeam Analysis. San Francisco Press, San Francisco, pp 241–242
Bulger RE, Beeuwkes R, Saubermann AJ (1981) Application of scanning electron microscopy to X-ray analysis of frozen-hydrated sections 3. Elemental content of cells in the rat renal papillary tip. J Cell Biol 88:274–280
Cameron IL (1983) Application of energy dispersive X-ray microanalysis for measuring ions in normal and tumour cells. J Ultrastruct Res 84:101–102
Civan MM, Hall TA, Gupta BL (1980) Microprobe study of toad urinary bladder in absence of serosal Kα. J. Membr Biol 55:187–202
Dow JAT, Gupta BL, Hall TA (1981) Microprobe analysis of Na, K, Cl, P, S, Ca, Mg and H2O in frozen-hydrated sections of anterior caeca of the locust,Schistocerca gregaria. J Insect Physiol 27:629–639
Ellis RA, Goertemiller CC, Stetson DL (1977) Significance of extensive ‘leaky’ cell junctions in the avian salt gland. Nature 268:555–556
Ernst SA, Ellis RA (1969) The development of surface specialization in the secretory epithelium of the avian salt gland in response to osmotic stress. J Cell Biol 40:305–321
Ernst SA, Mills JW (1977) Basolateral plasma membrane localization of ouabain-sensitive sodium transport sites in the secretory epithelium of the avian salt gland. J Cell Biol 75:74–94
Ernst SA, Hootman SR, Schreiber JH, Riddle CV (1981) Freeze-fracture and morphometric analysis of occluding junctions in rectal glands of elasmobranch fish. J Membr Biol 58:101–114
Eveloff J, Kinne R, Kinne-Saffran E, Murer H, Silva P, Epstein FH, Stoff J, Kinter WB (1978) Coupled sodium and chloride transport into plasma membrane vesicles prepared from dogfish rectal gland. Pflügers Arch 378:87–92
Fletcher GL, Stainer IM, Holmes WN (1967) Sequential changes in adenosinetriphosphatase activity and the electrolyte secretory capacity of the nasal salt glands of the duck (Anas platyrhynchos) during the period of adaptation to hypertonic saline. J Exp Biol 47:375–391
Frizzell RA, Field M, Schultz G (1979) Sodium-coupled chloride transport by epithelial tissues. Am J Physiol 236:F1-F8
Gilmore JP, Dietz J, Gilmore C, Zucker IH (1977) Evidence for a chloride pump in the salt gland of the goose. Comp Biochem Physiol 56A:121–126
Greger R, Schlatter E (1984) Mechanism of secretion in the rectal gland of spiny dogfish (Squalus acanthias). 1. Experiments in isolated in vitro perfused rectal gland tubules. Pflügers Arch 402:63–75
Gupta BL, Hall TA, Naftalin RJ (1978) Microprobe measurement of Na, K and Cl concentration profiles in epithelial cells and intercellular spaces of rabbit ileum. Nature 272:70–73
Hanwell A, Linzell JL, Peaker M (1971) Salt-gland secretion and blood flow in the goose. J Physiol 213:373–387
Hootman SR, Ernst SA (1981) Effect of methacholine on Naα pump activity and ion content of dispersed avian salt gland cells. Am J Physiol 241:R77-R86
Hughes MR (1970) Flow rate and cation concentration in salt gland secretion of the glaucous-winged gull,Larus glancescens. Comp Biochem Physiol 32:807–812
Hyatt AD, Marshall AT (1985) An alternative microdroplet method for quantitative X-ray microanalysis of biological fluids. Micron Microscopica Acta (in press)
Inoue T (1963) Nasal salt gland: Independence of salt and water transport. Science 142:1299–1300
Karnovsky MJ (1965) A formaldehyde-glutaladehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137A
Kaul R, Gerstberger R, Meyer JU (1981) Blood flow in salt glands of Pekin duck is proportional to salt secretion. Pflügers Arch 391:R25
Kaul R, Gerstberger R, Meyer JU, Simon E (1983) Salt gland blood flow in saltwater-adapted Pekin ducks. Microsphere measurement of the proportionality to secretion rate and investigation of controlling mechanisms. J Comp Physiol 149:457–462
Kendall MD, Warley A, Nicholson JK, Appleton TC (1983) X-ray microanalysis of proximal and distal tubule cells in the mouse kidney, and the influence of cadmium on the concentration of natural intracellular elements. J Cell Sci 62:319–338
Kirschner LB (1977) The sodium chloride excreting cells in marine vertebrates. In: Gupta BL, Moreton RB, Oschman JL, Wall BJ (ed) Transport of Ions and Water in Animals. Academic Press, London, pp 427–452
Kleinzeller A, Forrest JN, Cha CJ, Goldstein J, Booz G (1983) Cell solute composition and potassium effects in slices of the rectal gland of the dogfish shark (Squalus acanthias). J Comp Physiol B 155:145–153
Komnick H (1964) Elektronenmikroskopische Untersuchungen zur funktionellen Morphologie des Ionentransportes in der Salzdrüse vonLarus argentatus. Protoplasma 58:96–127
Marples BJ (1932) The structure and development of the nasal glands of birds. Proc Zool Soc Lond 1932:829–844
Marshall AT (1980) Quantitative X-ray microanalysis of frozen-hydrated bulk biological specimens. Scanning Electron Microsc 1980:335–348
Marshall AT (1981) Simultaneous use of EDS, windowless EDS, BE and SE detectors and digital real-time line scanning for the X-ray microanalysis of frozen-hydrated biological specimens. Scanning Electron Microsc 1981:327–343
Marshall AT (1982) Application of ϕ(ρζ) curves and a windowless detector to the quantitative X-ray microanalysis of frozen-hydrated bulk biological specimens. Scanning Electron Microsc 1982:243–260
Marshall AT (1984) The windowless energy dispersive X-ray detector: prospects for a role in biological X-ray microanalysis. Scanning Electron Microsc 1984:493–504
Marshall AT, Carde D (1984) Beryllium coating for biological X-ray microanalysis. J Microsc 134:113–116
Maunsbach AB (1966) The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. 1 Comparison of different perfusion methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res 15:242–282
Peaker M (1971a) Avian salt glands. Philos Trans R Soc Lond B 262:289–300
Peaker M (1971b) Intracellular concentrations of sodium, potassium and chloride in the salt gland of the domestic goose and their relation to the secretory mechanism. J Physiol 213:399–410
Peaker M, Linzell JL (1975) Salt Glands in Birds. Cambridge University Press. Cambridge
Peaker M, Stockley SJ (1973) Lithium secretion by the salt gland of the goose. Nature 243:297–298
Peaker M, Stockley SJ (1974) The effects of lithium and methacholine on the intracellular ionic composition of goose salt gland slices: relation to sodium and chloride transport. Experientia 30:158–159
Peaker M, Peaker SJ, Phillips JG, Wright A (1971) The effects of corticotrophin, glucose and potassium chloride on secretion by the nasal salt gland of the duckAnas platyrhynchos. J Endocrinol 50:293–299
Potts WTW, Oates K (1983) The ionic concentrations in the mitochondria-rich or chloride cell ofFundulus heteroclitus. J Exp Zool 227:349–359
Rick R, Dorge A, Macknight ADC, Leaf A, Thurau K (1978a) Electron microprobe analysis of the different epithelial cells of toad urinary bladder. J Membr Biol 39:257–271
Rick R, Dorge A, von Arnim E, Thurau K (1978b) Electron microprobe analysis of frog skin epithelium: evidence for a syncytial sodium transport compartment. J Membr Biol 39:313–331
Riddle CV, Ernst SA (1979) Structural simplicity of thezonula occludens in the electrolyte secreting epithelium of the avian salt gland. J Membr Biol 45:21–35
Roinel N, de Rouffignac Ch (1982) X-ray analysis of biological fluids: contribution of microdroplet technique to biology. Scanning Electron Microsc 1982:1155–1171
Roomans GM (1984) X-ray microanalysis of rat exorbital lacrimal gland. Scanning Electron Microsc 1984:889–895
Schmidt-Nielsen B (1976) Intracellular concentrations of the salt gland of the herring gullLarus argentatus. Am J Physiol 230:514–521
Schmidt-Nielsen K (1960) The salt-secreting gland of marine birds. Circulation 21:955–967
Silva P, Stoff J, Field M, Fine L, Forrest JN, Epstein FH (1977) Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol 233:F298-F306
Sokal RR, Rohlf FJ (1969) Biometry. Freeman and Company. San Francisco, pp 253–398
Thaysen JH (1979) The lacrimal gland. In: Gielbisch G (ed) Transport Organs. Springer. Berlin Heidelberg New York (Membrane transport in biology, vol 4B, pp 415–433)
Thesleff S, Schmidt-Nielsen K (1962) An electrophysiological study of the salt gland of the herring-gull. Am J Physiol 202:597–600
Thurau K, Beck F, Mason J, Dorge A, Rick R (1981) Inside the cell — an electron microprobe analysis of the renal tubular electrolyte concentrations. In: Macknight ADC, Leader JP (eds) Epithelial Ion and Water Transport. Raven Press, New York, pp 137–145
Trump BF, Ericsson JLE (1965) The effect of fixative solution on the ultrastructure of cells and tissues. Lab Invest 14:507–585
van Lennep EW, Young JA (1979) Salt glands. In: Giebisch G (ed) Transport Organs. Springer, Berlin Heidelberg New York (Membrane transport in biology, vol 4B, pp 675–692)
Welsh MJ, Smith PL, Frizzell RA (1983) Intracellular chloride activities in the isolated perfused shark rectal gland. Am J Physiol 245:F640-F644
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marshall, A.T., Hyatt, A.D., Phillips, J.G. et al. Isosmotic secretion in the avian nasal salt gland: X-ray microanalysis of luminal and intracellular ion distributions. J Comp Physiol B 156, 213–227 (1985). https://doi.org/10.1007/BF00695776
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00695776