Abstract
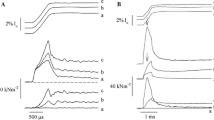
Here we report that mechanically skinned muscle preparations from slow amphibian muscle fibres become less sensitive to Ca2+ when the sarcomere length is increased beyond the value corresponding to optimum filament overlap. This effect is opposite to that observed in all other studies on vertebrate myofibrillar preparations from twitch (Endo, 1972a; 1972b; Moisescu & Thieleczek, 1979; Stephenson & Williams, 1982) and cardiac muscles (Fabiato & Fabiato, 1978). This finding shows that stretch-induced increase in Ca2+ sensitivity is not a general property of the contractile apparatus and suggests that differences in the ultrastructure between these muscle fibre types could be responsible for the opposite length effects. Furthermore, these results allow a more detailed understanding of the processes behind the stretch effects observed in intact slow and twitch amphibian muscle fibres (Lännergren, 1975).
Similar content being viewed by others
References
Close RI (1972) Dynamic properties of mammalian skeletal muscles. Physiol Rev 52: 129–197.
Costantin L, Podolsky RJ & Tice LW (1967) Calcium activation of frog slow muscle fibres. J Physiol 188: 261–271.
Elliott GF, Rome E & Spencer M (1970) A type of contraction hypothesis applicable to all muscles. Nature 226: 417–420.
Endo M (1972a) Stretch-induced increase in activation of skinned muscle fibres by calcium. Nature, New Biol 237: 211–213.
Endo M (1972b) Length dependence of activation of skinned muscle fibres by calcium. Cold Spring Harb Symp quant Biol 37: 505–510.
Endo M, Kitazawa T, Iino M & Kakuta Y (1976) In: Sugi H & Pollack GH (eds) Cross-bridge mechanism in muscle contraction. University Park Press, Baltimore, pp. 365–376.
Fabiato A & Fabiato F (1978) Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol 72: 667–699.
Gilly WF (1975) Slow fibres in the frog Cruralis muscle. Tissue & Cell 7: 203–210.
Gilly WF & Hui CS (1977) Contractile activation in slow and twitch muscle fibres of the frog. Nature 266: 186–188.
Hess A (1970) Vertebrate slow muscle fibres. Physiol Rev 50: 40–62
Julian FJ (1971) The effect of calcium on the forcevelocity relation of briefly glycinerated frog muscle fibres. J Physiol 218: 117–132.
Kirby AC, Godt RE & Gordon AM (1981) Hypertonic solutions decrease contractile force in frog slow muscle fibres by increasing internal ionic strength. J Gen Physiol 78: 20a.
Lännergren J (1975) The effect of stretch on potassium contracture tension in twitch and slow muscle fibres of Xenopus laevis. Acta Physiol Scand 95: 347–349.
Matsubara I & Elliott GF (1972) X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol 72: 657–669.
Miledi R, Parker I & Schalow G (1981) Calcium transients in normal and denervated slow muscle fibres of the frog. J Physiol 318: 191–206.
Miller A & Woodhead-Galloway J (1971) Long range forces in muscle. Nature 229: 470–473.
Moisescu DG (1973) Interfilament forces in striated muscle. Bull Math Biol 35: 565–575.
Moisescu DG (1976) Kinetics of reaction in calciumactivated skinned muscle fibres. Nature 262: 610–613.
Moisescu DG & Thieleczek R (1978) Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol 275: 241–262.
Moisescu DG & Thieleczek R (1979) Sarcomere length effects on the Sr++-and Ca++-activation curves in skinned frog muscle fibres. Biochem Biophys Acta 546: 64–76.
Page SG (1965) A comparison of the fine structure of frog slow and twitch muscle fibres. J Cell Biol 26: 477–497.
Simmons RM (1981) Mechanical rate constants in frog muscle skinned fibres at various MgATP concentrations. J Gen Physiol 78: 3–4a.
Stefani E & Uchitel OD (1976) Potassium and calcium conductance in slow muscle fibres of the toad. J Physiol 255: 435–448.
Stephenson DG & Williams DA (1981) Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol 317: 281–302.
Stephenson DG & Williams DA (1982) Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol 333: 637–655.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stephenson, D.G., Williams, D.A. Slow amphibian muscle fibres become less sensitive to Ca2+ with increasing sarcomere length. Pflugers Arch. 397, 248–250 (1983). https://doi.org/10.1007/BF00584366
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584366