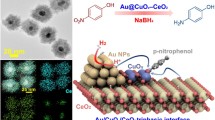
Abstract
Metal oxide hollow structures are of great interest in many current and emerging areas of technology. This paper presents a facile and controlled protocol for the synthesis of Al-doped CeO2 hollow-shell spheres (CHS), where the dopant confers enhanced stability and activity to the material. These Al-doped CeO2 hollow-shell spheres (ACHS) possess a controllable shell number of up to three, where the sizes of the exterior, middle, and interior spheres were about 250‒100 nm,150‒50 nm, and 40‒10 nm, respectively, and the average shell thickness was ~15 nm. The thermal stability of the ACHS structure was enhanced by the homogeneous incorporation of Al atoms, andmore active oxygen species were present compared with those in the non-doped congener. Au NPs supported on ACHS (Au/ACHS) showed superior catalytic performance for the reduction of p-nitrophenol. For the same Au NP content, the reaction rate constant (k) of the Au/ACHS was nearly twice that of the non-doped Au/CHS, indicating that Al doping is promising for improving the performance of inert or unstable oxides as catalyst supports.
摘要
本文利用多孔碳球为模板, 通过竞争吸附控制煅烧法制备了铝掺杂的氧化铈多壳层空心球材料. 通过XRD、SEM、TEM对所得材料的结构、表面形貌以及热稳定性能进行了表征, 结果表明该方法制备的不同掺杂比例的多壳层空心球均一分散, 铝原子被均匀地分布到氧化铈的晶格中, 使其热稳定性得到提高. XPS结果表明铝的掺杂大大提高了材料中三价铈和表面氧空位的比例, 所以当其被作为催化剂载体时, 负载的金纳米粒子更加分散且与载体结合作用更强. 将该催化剂应用于硝基苯酚加氢反应, 其催化活性比未掺杂铝的样品提高了一倍.本文研究结果表明铝掺杂可以有效提高氧化铈催化剂的稳定性和活性; 竞争吸附控制煅烧法是制备空心掺杂材料的一种简单实用的方法.
Similar content being viewed by others
References
Sun C, Li H, Chen L. Nanostructured ceria-based materials: synthesis, properties, and applications. Energ Environ Sci, 2012, 5: 8475–8505
Lawrence NJ, Brewer JR, Wang L, et al. Defect engineering in cubic cerium oxide nanostructures for catalytic oxidation. Nano Lett, 2011, 11: 2666–2671
Branda MM, Loschen C, Neyman KM, et al. Atomic and electronic structure of cerium oxide stepped model surfaces. J Phys Chem C, 2008, 112: 17643–17651
Zhang C, Michaelides A, Jenkins SJ. Theory of gold on ceria. Phys Chem Chem Phys, 2011, 13: 22–33
Zhao K, Qi J, Yin H, et al. Efficient water oxidation under visible light by tuning surface defects on ceria nanorods. J Mater Chem A, 2015, 3: 20465–20470
Skorodumova NV, Simak SI, Lundqvist BI, et al. Quantum origin of the oxygen storage capability of ceria. Phys Rev Lett, 2002, 89: 166601
Pu ZY, Liu XS, Jia AP, et al. Enhanced activity for CO oxidation over Pr- and Cu-doped CeO2 catalysts: effect of oxygen vacancies. J Phys Chem C, 2008, 112: 15045–15051
Kehoe AB, Scanlon DO, Watson GW. Role of lattice distortions in the oxygen storage capacity of divalently doped CeO2. Chem Mater, 2011, 23: 4464–4468
Li C, Xin Q, Guo X. Surface oxygen species and their reactivities in the mild oxidation of ethylene on cerium oxide studied by FT-IR spectroscopy. Catal Lett, 1992, 12: 297–305
Trovarelli A. Catalytic properties of ceria and CeO2-containingmaterials. Catal Rev, 1996, 38: 439–520
Nolan M. Charge compensation and Ce3+ formation in trivalent doping of the CeO2 (110) surface: the key role of dopant ionic radius. J Phys Chem C, 2011, 115: 6671–6681
Yuzhakova T, Rakić V, Guimon C, et al. Preparation and characterization of Me2O3−CeO2 (Me=B, Al, Ga, In) mixed-oxide catalysts. Chem Mater, 2007, 19: 2970–2981
Pijolat M, Prin M, Soustelle M, et al. Thermal stability of doped ceria: experiment and modelling. Faraday Trans, 1995, 91: 3941–3948
Ilieva-Gencheva L, Pantaleo G, Mintcheva N, et al. Nano-structured gold catalysts supported on CeO2 and CeO2-Al2O3 for NOx reduction by CO: effect of catalyst pretreatment and feed composition. J Nanosci Nanotech, 2008, 8: 867–873
Yao H. Ceria in automotive exhaust catalysts I. Oxygen storage. J Catal, 1984, 86: 254–265
Lou XWD, Archer LA, Yang Z. Hollow micro-/nanostructures: synthesis and applications. Adv Mater, 2008, 20: 3987–4019
Zhang H, Zhu Q, Zhang Y, et al. One-pot synthesis and hierarchical assembly of hollow Cu2O microspheres with nanocrystalscomposed porousmultishell and their gas-sensing properties. Adv Funct Mater, 2007, 17: 2766–2771
Lee JH. Gas sensors using hierarchical and hollowoxide nanostructures: overview. Sensors Actuators B-Chem, 2009, 140: 319–336
Cui P, Wang J, Wang Z, et al. Bismuth oxychloride hollow microspheres with high visible light photocatalytic activity. Nano Res, 2016, 9: 593–601
Wang Z, Zhou L, David Lou XW. Metal oxide hollow nanostructures for lithium-ion batteries. Adv Mater, 2012, 24: 1903–1911
Li S, Li A, Zhang R, et al. Hierarchical porous metal ferrite ball-in-ball hollow spheres: general synthesis, formation mechanism, and high performance as anodematerials for Li-ion batteries. Nano Res, 2014, 7: 1116–1127
An K, Hyeon T. Synthesis and biomedical applications of hollow nanostructures. Nano Today, 2009, 4: 359–373
Qi J, Lai X, Wang J, et al. Multi-shelled hollow micro-/nanostructures. Chem Soc Rev, 2015, 44: 6749–6773
Liu H, Ma H, Joo J, et al. Contribution of multiple reflections to light utilization efficiency of submicron hollow TiO2 photocatalyst. Sci China Mater, 2016, 59: 1017–1026
Wang J, Tang H, Zhang L, et al. Multi-shelledmetal oxides prepared via an anion-adsorption mechanism for lithium-ion batteries. Nat Energ, 2016, 1: 16050
Zhao K, Qi J, Zhao S, et al. Multiple Au cores in CeO2 hollow spheres for the superior catalytic reduction of p-nitrophenol. Chin J Catal, 2015, 36: 261–267
Keilitz J, Schwarze M, Nowag S, et al. Homogeneous stabilization of Pt nanoparticles in dendritic core-multishell architectures: application in catalytic hydrogenation reactions and recycling. Chem-Cat Chem, 2010, 2: 863–870
Sun X, Li Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew Chem Int Ed, 2004, 43: 597–601
Xu P, Yu R, Ren H, et al. Hierarchical nanoscale multi-shell Au/CeO2 hollow spheres. Chem Sci, 2014, 5: 4221–4226
Wang Y, Wang F, Chen Y, et al. Enhanced photocatalytic performance of ordered mesoporous Fe-doped CeO2 catalysts for the reduction of CO2 with H2O under simulated solar irradiation. Appl Catal B-Environ, 2014, 147: 602–609
Larachi F, Pierre J, Adnot A, et al. Ce 3d XPS study of composite CexMn1−x O2−y wet oxidation catalysts. Appl Surface Sci, 2002, 195: 236–250
Huang X, Beck MJ. Size-dependent appearance of intrinsic Ox q “activated oxygen” molecules on ceria nanoparticles. Chem Mater, 2015, 27: 5840–5844
Dos Santos ML, Lima RC, Riccardi CS, et al. Preparation and characterization of ceria nanospheres bymicrowave-hydrothermal method. Mater Lett, 2008, 62: 4509–4511
Huang X, Guo C, Zuo J, et al. An assembly route to inorganic catalytic nanoreactors containing sub-10-nm gold nanoparticles with anti-aggregation properties. Small, 2009, 5: 361–365
Panigrahi S, Basu S, Praharaj S, et al. Synthesis and size-selective catalysis by supported gold nanoparticles: study on heterogeneous and homogeneous catalytic process. J Phys Chem C, 2007, 111: 4596–4605
Zhang C, Michaelides A, King DA, et al. Structure of gold atoms on stoichiometric and defective ceria surfaces. J Chem Phys, 2008, 129: 194708–194708
Zhang C, Michaelides A, King DA, et al. Anchoring sites for initial au nucleation on CeO2 {111}: O vacancy versus Ce vacancy. J Phys Chem C, 2009, 113: 6411–6417
Zhang J, Chen G, Guay D, et al. Highly active PtAu alloy nanoparticle catalysts for the reduction of 4-nitrophenol. Nanoscale, 2014, 6: 2125–2130
Ta N, Liu JJ, Chenna S, et al. Stabilized gold nanoparticles on ceria nanorods by strong interfacial anchoring. J Am Chem Soc, 2012, 134: 20585–20588
Sun Z, Liao T, Kou L. Strategies for designing metal oxide nanostructures. Sci China Mater, 2017, 60: 1–24
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51472025 and 21671016) and Beijing Nova Programme Interdisciplinary Cooperation Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions Wang Z designed and engineered the samples; Jiang S and Li Y performed the experiments;Wang Z wrote the paper with support from Jiang S. All authors contributed to the general discussion.
Conflict of interest The authors declare that they have no conflict of interest.
Supplementary information Supporting data are available in the online version of the paper.
Zumin Wang received his BE degree from the School of Metallurgical and Ecological Engineering, the University of Science and Technology, Beijing (USTB) in 2012. He is currently studying for his PhD degree under the supervision of Prof. Ranbo Yu from the Laboratory of Physical Chemistry of Materials Preparation at the Department of Physical Chemistry, USTB.His current research interests are focused on the design and synthesis ofmicro-/nanostructured functional inorganic nanomaterials for catalysis.
Shuaiyu Jiang received his BE degree from the University of Science and Technology Liaoning (2013) and MSc from the USTB (2016). He is currently a PhD candidate at the Griffith University. His current research interest is focused on the synthesis of micro-/nanostructured photocatalyst.
Ranbo Yu received her BSc and MSc degrees at Jilin University (1994 and 1997) and PhD at Yamanashi University (2002). As a postdoctoral researcher she worked in Kyoto University (JSPS fellow) and the University of Houston in 2002 and 2003. She currently holds a professor position at the Department of Physical Chemistry, USTB. Her research interests focus on the development of micro-/nanostructured functional inorganic materials and their applications in catalysis.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Z., Jiang, S., Li, Y. et al. Highly active CeO2 hollow-shell spheres with Al doping. Sci. China Mater. 60, 646–653 (2017). https://doi.org/10.1007/s40843-017-9042-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9042-0