Abstract
Purpose of Review
It has long been considered that tolerance in a transplant recipient is a binary all-or-none state: either the graft is accepted without immunosuppression identifying the recipient as tolerant, or the recipient rejects the graft and is not tolerant. This tolerance paradigm, however, does not accurately reflect data emerging from animal models and patients and requires revision.
Recent Findings
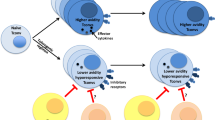
It is becoming appreciated that there may be different gradations in the quality of transplantation tolerance based on underlying cellular mechanisms of immunological tolerance, and that individuals may enhance the robustness of their state of transplant tolerance by strengthening or combining different cellular mechanisms. Furthermore, evidence suggests that even if tolerance is lost, the loss may be only temporary, and in some circumstances, tolerance can be restored.
Summary
Shifting our focus from an all-or-nothing tolerance paradigm to one with many shades may help us better understand how tolerance operates, and how this state may be tracked and enhanced for better patient outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15:2921–30. https://doi.org/10.1111/ajt.13347.
de Vries VC, Wasiuk A, Bennett KA, et al. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–80. https://doi.org/10.1111/j.1600-6143.2009.02755.x.
• Miller ML, Daniels MD, Wang T, et al. Tracking of TCR-Tg T cells reveals multiple mechanisms maintain cardiac transplant tolerance in mice. Am J Transplant. 2016; https://doi.org/10.1111/ajt.13814. Study showing that robust tolerance is maintained by multiple additive/redundant cellular tolerance mechanisms.
Iida S, Suzuki T, Tanabe K, et al. Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection. Am J Transplant. 2013;13:2268–79. https://doi.org/10.1111/ajt.12342.
Zhang L, Chen X, Liu X, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Investig. 2013;123:1999–2010. https://doi.org/10.1172/JCI63980.
Ali JM, Negus MC, Conlon TM, et al. Diversity of the CD4 T cell alloresponse: the short and the long of it. Cell Rep. 2016;14:1232–45. https://doi.org/10.1016/j.celrep.2015.12.099.
Wang T, Ahmed EB, Chen L, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10:1524–33. https://doi.org/10.1111/j.1600-6143.2010.03066.x.
Wang T, Chen L, Ahmed EM, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991–9.
Ahmed EB, Wang T, Daniels M, et al. IL-6 induced by Staphylococcus aureus infection prevents the induction of skin allograft acceptance in mice. Am J Transplant. 2011;11:936–46. https://doi.org/10.1111/j.1600-6143.2011.03476.x.
Welsh RM, Markees TG, Woda BA, et al. Virus-induced abrogation of transplantation tolerance induced by donor-specific transfusion and anti-CD154 antibody. J Virol. 2000;74:2210–8.
•• Young JS, Daniels MD, Miller ML, et al. Erosion of transplantation tolerance after infection. Am J Transplant. 2017;17:81–90. https://doi.org/10.1111/ajt.13910. A study showing that a severe infection can erode transplantation tolerance long-term even in animals in which it does not cause graft loss.
•• Miller ML, Daniels MD, Wang T, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015;6:7566. https://doi.org/10.1038/ncomms8566. A study showing that the memory of tolerance can dominate over the memory of transplant rejection triggered by an infection, albeit the restored tolerance is eroded when compared to the robust tolerance prior to infection .
Jiang X, Sun W, Guo D, et al. Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+CD25+ regulatory T cells. Surgery. 2011;149:336–46. https://doi.org/10.1016/j.surg.2010.08.012.
Qin S, Cobbold SP, Pope H, et al. “Infectious” transplantation tolerance. Science. 1993;259:974–7.
Kendal AR, Chen Y, Regateiro FS, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011;208:2043–53. https://doi.org/10.1084/jem.20110767.
Graca L, Thompson S, Lin C-Y, et al. Both CD4+CD25+ and CD4+CD25− regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558–65. https://doi.org/10.4049/jimmunol.168.11.5558.
Schroeder G, Risch K, Kotsch K, et al. FTY720 prevents anti-CD4 mAb-induced tolerance but cannot reverse established tolerance in a rat kidney transplantation model. Am J Transplant. 2004;4:863–71. https://doi.org/10.1111/j.1600-6143.2004.00442.x.
Scalea JR, Okumi M, Villani V, et al. Abrogation of renal allograft tolerance in MGH miniature swine: the role of intra-graft and pripheral factors in long-term tolerance. Am J Transplant. 2014;14:2001–10. https://doi.org/10.1111/ajt.12816.
Bigenzahn S, Blaha P, Koporc Z, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–47.
Besançon A, Baas M, Goncalves T, et al. The induction and maintenance of transplant tolerance engages both regulatory and anergic CD4(+) T cells. Front Immunol. 2017;8:218. https://doi.org/10.3389/fimmu.2017.00218.
Baas M, Besançon A, Goncalves T, et al. TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. elife. 2016; https://doi.org/10.7554/eLife.08133.
Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007;179:5204–10.
Koehn BH, Ford ML, Ferrer IR, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008;181:5313–22.
Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–45. https://doi.org/10.1016/j.ajpath.2010.12.024.
• Yamada Y, Nadazdin O, Boskovic S, et al. Repeated injections of IL-2 break renal allograft tolerance induced via mixed hematopoietic chimerism in monkeys. Am J Transplant. 2015;15:3055–66. https://doi.org/10.1111/ajt.13382. The authors provide evidence of breaking of transplantation tolerance in non-human primates, and of its spontaneous restoration following cessation of IL-2 administration .
Leventhal J, Abecassis M, Miller J, et al. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. https://doi.org/10.1126/scitranslmed.3003509.
Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. https://doi.org/10.1056/NEJMoa071074.
Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–8. https://doi.org/10.1056/NEJMoa074191.
Morris H, DeWolf S, Robins H, et al. Tracking donor-reactive T cells: evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. 2015;7:272ra10. https://doi.org/10.1126/scitranslmed.3010760.
Haspot F, Fehr T, Gibbons C, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149–55. https://doi.org/10.1182/blood-2007-12-127449.
Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–611. https://doi.org/10.1111/ajt.12731.
Massart A, Pallier A, Pascual J, et al. The DESCARTES-Nantes survey of kidney transplant recipients displaying clinical operational tolerance identifies 35 new tolerant patients and 34 almost tolerant patients. Nephrol Dial Transplant. 2016;31:1002–13. https://doi.org/10.1093/ndt/gfv437.
Sagoo P, Perucha E, Sawitzki B, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–61. https://doi.org/10.1172/JCI39922.
Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–47. https://doi.org/10.1172/JCI39933.
Chenouard A, Chesneau M, Bui Nguyen L, et al. Renal operational tolerance is associated with a defect of blood Tfh cells that exhibit impaired B cell help. Am J Transplant. 2016; https://doi.org/10.1111/ajt.14142.
Braza F, Dugast E, Panov I, et al. Central role of CD45RA- Foxp3hi memory regulatory T cells in clinical kidney transplantation tolerance. J Am Soc Nephrol. 2015;26:1795–805. https://doi.org/10.1681/ASN.2014050480.
Asare A, Kanaparthi S, Lim N, et al. B Cell receptor genes associated with tolerance identify a cohort of immunosuppressed patients with improved renal allograft graft function. Am J Transplant. 2017; https://doi.org/10.1111/ajt.14283.
Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, et al. Biomarkers of tolerance in kidney transplantation: are we predicting tolerance or response to immunosuppressive treatment? Am J Transplant. 2016;16:3443–57. https://doi.org/10.1111/ajt.13932.
Bottomley MJ, Chen M, Fuggle S, et al. Application of operational tolerance signatures are limited by variability and type of immunosuppression in renal transplant recipients: a cross-sectional study. Transplant Direct. 2017;3:e125. https://doi.org/10.1097/TXD.0000000000000638.
Bohne F, Martínez-Llordella M, Lozano J-J, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012;122:368–82. https://doi.org/10.1172/JCI59411.
• Benítez C, Londoño M-C, Miquel R, et al. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824–35. https://doi.org/10.1002/hep.26426. In one of the first studies to prospectively wean patients from immunosuppression, the authors find that episodes of acute rejection do not preclude tolerance from later developing.
•• Brouard S, Pallier A, Renaudin K, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12:3296–307. https://doi.org/10.1111/j.1600-6143.2012.04249.x. This study provides evidence that spontaneous tolerance in human patients is not always permanent.
Tryphonopoulos P, Ruiz P, Weppler D, et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation. 2010;90:1556–61. https://doi.org/10.1097/TP.0b013e3182003db7.
Mazariegos GV, Sindhi R, Thomson AW, Marcos A. Clinical tolerance following liver transplantation: long term results and future prospects. Transpl Immunol. 2007;17:114–9. https://doi.org/10.1016/j.trim.2006.09.033.
Barbi J, Pardoll D, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115–39. https://doi.org/10.1111/imr.12172.
Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6.
Nish SA, Schenten D, Wunderlich FT, et al. T cell-intrinsic role of IL-6 signaling in primary and memory responses. elife. 2014;3:e01949. https://doi.org/10.7554/eLife.01949.
Brown IE, Blank C, Kline J, et al. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–9.
• Schietinger A, Delrow JJ, Basom RS, et al. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–7. https://doi.org/10.1126/science.1214277. In this work, the authors identify an epigenetically-programmed T cell-intrinsic tolerant state that can be reversed and later spontaneously restored.
Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol. 2008;180:5816–25.
Ardolino M, Azimi CS, Iannello A, et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J Clin Invest. 2014;124:4781–94. https://doi.org/10.1172/JCI74337.
Farkas AM, Finn OJ. Novel mechanisms underlying the immediate and transient global tolerization of splenic dendritic cells after vaccination with a self-antigen. J Immunol. 2014;192:658–65. https://doi.org/10.4049/jimmunol.1301904.
Farkas AM, Marvel DM, Finn OJ. Antigen choice determines vaccine-induced generation of immunogenic versus tolerogenic DC that are marked by differential expression of pancreatic enzymes. J Immunol. 2013;190:3319–27. https://doi.org/10.4049/jimmunol.1203321.
Yang Y, Huang C-T, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–15. https://doi.org/10.1038/ni1059.
Horkheimer I, Quigley M, Zhu J, et al. Induction of type I IFN is required for overcoming tumor-specific T-cell tolerance after stem cell transplantation. Blood. 2009;113:5330–9. https://doi.org/10.1182/blood-2008-05-155150.
• Legoux FP, Lim J-B, Cauley AW, et al. CD4(+) T Cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. 2015;43:896–908. https://doi.org/10.1016/j.immuni.2015.10.011. Demonstration that self-tolerance is also functionally graded in its robustness based on the mechanism of tolerance .
Malhotra D, Linehan JL, Dileepan T, et al. Tolerance is established in polyclonal CD4+ T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol. 2016;17:187–95. https://doi.org/10.1038/ni.3327.
Goding SR, Wilson KA, Xie Y, et al. Restoring immune function of tumor-specific CD4+ T cells during recurrence of melanoma. J Immunol. 2013;190:4899–909. https://doi.org/10.4049/jimmunol.1300271.
Wood LM, Paterson Y. Attenuated Listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4:51. https://doi.org/10.3389/fcimb.2014.00051.
Funding
M.L.M. was funded by American Heart Association predoctoral fellowships (13PRE14550022 and 15PRE22180007), a Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), and a Howard Hughes Medical Institute Med-into-Grad Program training grant (56006772). The work was also supported by National Institutes of Health P01AI-97113 to A.S.C. and M.-L.A.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Anita Chong and Maria-Luisa declare grants from the National Institutes of Health during the conduct of this study.
Michelle Miller reports grants from National Institutes of Health, the American Heart Association, and the Howard Hughes Medical Institute during the conduct of the study.
Human and Animal Rights and Informed Consent
Reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Immunology
Rights and permissions
About this article
Cite this article
Miller, M.L., Chong, A.S. & Alegre, ML. Fifty Shades of Transplantation Tolerance: Beyond a Binary Tolerant/Non-Tolerant Paradigm. Curr Transpl Rep 4, 262–269 (2017). https://doi.org/10.1007/s40472-017-0166-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-017-0166-5