Abstract
Background
Current recombinant human growth hormone (r-hGH) replacement therapy involves long-term daily subcutaneous injections to treat growth hormone deficiency (GHD) in children and adults. Daily r-hGH injections can be burdensome, often resulting in poor treatment compliance. Clinical outcome assessments (COAs) can capture the burden of these injections from the patient (and caregiver) perspective and may demonstrate the benefit of a less-frequent r-hGH injection regimen, which may ultimately improve treatment compliance and long-term outcomes.
Objective
To address this knowledge gap, qualitative research was conducted to inform the development of a new Life Interference Questionnaire for Growth Hormone Deficiency (LIQ-GHD), designed to measure the experiences of patients taking r-hGH GHD injections. A second objective was to evaluate the hypothesized factor structure and preliminary performance of the LIQ-GHD in a cross-sectional observational study.
Methods
An empirical literature review and expert advice meetings were conducted to inform development of the draft LIQ-GHD (pediatric and adult versions). In-person concept elicitation and cognitive debriefing interviews were conducted with GHD patients (and patient dyads including caregivers) to explore and confirm concept coverage and evaluate respondents’ ability to understand the questionnaire. The draft LIQ-GHD was then tested in a cross-sectional field study involving pediatric and adult patients receiving daily r-hGH injections for GHD. The factor structure, reliability, and validity were analyzed for the overall sample and for pediatric, adolescent, and adult subgroups.
Results
Results from the literature review and input from six experts were used to develop and refine the LIQ-GHD, with content covering pen ease of use; regimen convenience; life interference due to regimen; benefit/satisfaction/willingness to continue treatment; regimen choice/preference; intent to comply with regimen; injection-related signs/symptoms; and reasons for missed injections. Twenty-one patient interviews confirmed comprehensive concept coverage and patient/caregiver comprehension of the LIQ-GHD. A total of 224 patients (n = 70 children/caregiver dyads, n = 79 adolescents/caregiver dyads, n = 75 adults) participated in the field study. While most items showed floor effects, confirmatory factor analysis fit statistics were good for the overall sample (root mean square error of approximation = 0.07, comparative fit index = 0.98) and for the full pediatric sample after dropping co-dependent questions from the model. Cronbach’s alpha (α) ranged from 0.746 to 0.905 and intra-class correlation coefficients ranged from 0.761 to 0.918 for the overall sample on LIQ-GHD domains. Scores correlated as predicted with an existing criterion measure in the overall sample and LIQ-GHD domain scores distinguished known groups as expected.
Conclusions
The LIQ-GHD is a new COA for the measurement of r-hGH injection treatment burden. This research provides evidence supporting its content validity, hypothesized factor structure, score reliability, and construct validity in pediatric and adult populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Life Interference Questionnaire for Growth Hormone Deficiency (LIQ-GHD) is a newly developed questionnaire to evaluate the burden of growth hormone injections in children (and their caregivers) and adults. |
A robust scientific instrument development approach was followed, yielding a tool with evidence of score reliability and validity. |
Additional analysis and on-going use of this questionnaire in clinical trials is recommended. |
1 Introduction
The prevalence of pediatric growth hormone deficiency (GHD) is estimated at approximately 1:4000 to 1:10,000 [1,2,3,4]. The most apparent feature of GHD in children is growth failure or growth restriction. Metabolic consequences include impaired lipid metabolism, impaired protein synthesis, and impaired bone mineralization. GHD in children may also impact psychosocial development, resulting in poor self-image and social isolation. Short stature is also linked to a decrease in quality of life (QoL) [5,6,7].
Recombinant human growth hormone (r-hGH) replacement therapy has been available to safely and effectively treat adults and children with GHD for the last several decades to (i) increase height during childhood; (ii) attain adult height targets; (iii) minimize adverse events; (iv) achieve cost-effective treatment [8]; and (v) improve QoL (since taller height in children is associated with a higher QoL) [9].
Therapy with r-hGH involves administration of subcutaneous injections given daily over the long-term (e.g., ≥ 5 years), until final height has been attained. Given the duration of r-hGH treatment, patients often do not optimally comply with treatment [10, 11], and non-compliance has been shown to be the most common cause of reduced height velocity in children [11,12,13]. Studies have also demonstrated a reduction in adherence over time [13].
There are many reasons for non-compliance, including complexity of regimen, difficulty in understanding potential treatment benefits, long-term treatment duration, perception of injections as painful, adolescence, and living in a busy, chaotic household [14,15,16,17]. However, the current literature suggests that little information exists describing the burden of administering daily growth hormone (GH) injections over the long-term. Further, there are currently no clinical outcome assessment (COA) measures available that can adequately assess the impacts and burden of r-hGH injections.
Since the administration of the injection to children often involves both the caregiver and patient, COA measures designed to evaluate r-hGH injection treatment burden should include feedback from both parties. A dyad approach to COA measurement (i.e., where the patient and caregiver read and answer questions together) may be useful to overcome concordance issues that arise in obtaining information from the caregiver or patient separately [18]. Although little research has been conducted to evaluate dyad-administered COAs, a study by Ungar et al. [19] suggested that a dyad approach could help children by enabling them to answer questionnaire items accurately, providing additional support in terms of comprehension or recall problems.
Thus, the overall goal of this research was to develop a new questionnaire, the Life Interference Questionnaire for Growth Hormone Deficiency (LIQ-GHD), to assess r-hGH injection treatment burden. The LIQ-GHD addresses an important measurement gap and offers versions for both adult and child–parent dyad administration.
The research summarizing the development and preliminary psychometric evaluation of the LIQ-GHD is presented here.
2 Objectives
The specific objectives of this research were to (i) establish evidence of content validity for the LIQ-GHD through qualitative research activities; and (ii) evaluate the hypothesized factor structure and preliminary score psychometric performance in a cross-sectional observational study.
3 Methods
As described in this section, the LIQ-GHD was developed and evaluated in a manner consistent with guidance provided by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) “Pediatric Patient-Reported Outcome Instruments for Research to Support Medical Product Labeling: Report of the ISPOR PRO Good Research Practices for the Assessment of Children and Adolescents Task Force” [20,21,22].
3.1 Establishing Evidence of Content Validity
‘Content validity’ refers to the extent to which a questionnaire measures concepts of interest in ways that are relevant and understandable to patients, comprehensive, and appropriate for the questionnaire’s intended context of use [20]. The target patient population for the developed questionnaire was adults (aged ≥ 25 years) as well as adolescents (aged 12–17 years) and children (aged 3–11 years). The period of 18–24 years of age is generally viewed as a ‘transition period’ in GHD, during which individuals with pediatric-onset GHD experience a slowed growth rate as they approach their adult height [25,26,27]. In order to avoid confounding the study results, and to clearly distinguish between pediatric and adult GHD populations, individuals aged 18–24 years were not included in the study population.
With this in mind, evidence for the content validity of the LIQ-GHD was developed through a targeted review of empirical literature, advice meetings with clinical experts, and qualitative interviews with adults (age ≥ 25 years) and children (age 3–17 years) taking r-hGH injections for GHD.
3.1.1 Targeted Review of Empirical Literature
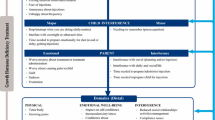
A review of empirical literature was conducted in March 2016 using PubMed (https://www.ncbi.nlm.nih.gov/pubmed). Searches were conducted to identify publications with potential relevance to dosing frequency and injection issues related to hGH, and other therapeutic areas with a similar treatment regimen in adult and pediatric populations. A supplemental literature search was also conducted through review of the reference lists of publications reviewed in the original search, conference proceedings, and Google and Google Scholar. A literature-focused conceptual model [23] was developed to represent r-hGH injection burden concepts and ultimately inform the selection of measurement concepts for the LIQ-GHD.
3.1.2 Questionnaire Modification and Development
A full-day, in-person meeting was convened by the LIQ-GHD developers, including the study sponsor, health outcomes researchers, GHD clinical experts, and COA measurement scientists. Results from the empirical literature review were reviewed and used to incorporate concepts and items from two existing questionnaires (the Injection Pen Assessment Questionnaire [IPAQ] [18] and the Bother, Satisfaction, and Willingness to Continue [BSW] Questionnaire [24]) and to develop new items for inclusion in the LIQ-GHD. Response scales were developed to be appropriate to the concept measured, consistent among like items, sensitive enough to capture change, and easy to use. Items assessing ease of treatment and satisfaction used a 5-point Likert-type scale to capture the entire response range from easy/satisfied to difficult/dissatisfied. Items assessing frequency of experience used a 5-point verbal response scale, while items assessing sign or symptom severity used an 11-point numeric rating scale.
3.1.3 Advice Meetings with Clinical Experts
One-on-one, 60-min advice meetings were conducted by telephone with clinical experts from the USA and European Union (EU). During these meetings, experts were asked to describe reported patient experiences (patients as well as caregivers) related to daily r-hGH injections and their perspective on the injection-related burdens that patients experience. Experts were also asked to provide feedback on the relevance and comprehensiveness of the draft LIQ-GHD.
3.1.4 Translatability Assessment
Following advice meetings with clinical experts, and before conducting qualitative patient interviews, the translatability of the draft LIQ-GHD was assessed by an independent translation provider (TransPerfect; http://www.transperfect.com/), in order to identify any words or terms that may pose difficulty in translation. Any wording revisions made to the LIQ-GHD during the qualitative interviews were subsequently submitted for translatability assessment.
3.1.5 Qualitative Interviews with Patients and Caregivers
A series of in-person, 90-min qualitative interviews were conducted with patients (and, when appropriate, their caregivers) in the USA diagnosed with GHD and receiving daily rhGH injections. Ethics approval was received from Quorum Review. Participants were recruited from four clinical sites, and included adults (aged ≥ 25 years) and adolescents (aged 12–17 years) and children (aged 3–11 years) with their caregivers. The qualitative interviews comprised two parts: (i) concept elicitation during which participants were asked open-ended questions about the r-hGH injection treatment burden and impacts they experienced due to r-hGH injections; and (ii) debriefing during which participants were asked to provide feedback on the LIQ-GHD, to assess its relevance (i.e., does it assess concepts relevant and important to participants?), comprehensiveness (i.e., does it omit any relevant concepts?), and comprehensibility (i.e., can participants understand the LIQ-GHD as intended by the developers and select meaningful responses?). Interviews were conducted in three waves to allow time for questionnaire revisions, if needed. Participant compensation was distributed upon interview completion.
3.1.6 Questionnaire Revision
Based on the translatability assessments and feedback from patients with GHD (and their caregivers), further updates were made to both LIQ-GHD versions: wording and organization were revised, and some concepts for measurement were added prior to quantitative testing.
3.2 Evaluation of Hypothesized Factor Structure and Score Psychometric Performance
An online, cross-sectional observational study was conducted to evaluate the LIQ-GHD hypothesized factor structure and score psychometric performance. Participants were clinician-diagnosed adult and pediatric (child and adolescent) patients receiving daily r-hGH injections for GHD, recruited from eight endocrinology clinics. Ethical approval was provided by Quorum Review and, where applicable, local ethical review boards. Consented participants completed the LIQ-GHD online at home. Questions related to the shared injection experience were completed by child and adolescent patients together with their caregiver (dyad administration). To enable test–retest analysis, the LIQ-GHD was completed twice in the same session with a brief interval between administrations, during which time participants completed a demographic form and the Self-Injection Assessment Questionnaire (SIAQ) [28]. Participant compensation was distributed upon questionnaire completion.
Analyses were conducted for the overall study sample and age-related subgroups (i.e., children, adolescents, and adults).
3.2.1 Factor Structure
Confirmatory factor analysis (CFA) was conducted to confirm the hypothesized factor structure of the LIQ-GHD (as summarized in Table 1 and presented in Fig. 1). The LIQ-GHD measures six hypothesized domains: Pen Ease of Use (PEoU; five items), Ease of Injection Schedule (EoIS; two items), Patient Life Interference (LI; five- or seven-item version), Satisfaction and Willingness to Continue Treatment (WtC; two items), Missed Injections (two items), and Injection Signs and Symptoms (SS; four items). Additional domains were hypothesized for the LIQ-GHD pediatric version: Injection Signs Reported by Caregiver (CS; two items), Caregiver Life Interference (CLI; five- or seven-item version), and Family Life Interference (FLI; five- or six-item version). CFA models were run for the overall sample and by age subgroup. Model fit was assessed with the comparative fit index (CFI) and root mean square error of approximation (RMSEA). CFI values can range between 0 and 1, with higher values indicative of better fit, and CFI > 0.95 is considered to be a good fit. RMSEA values ranging from 0.08 to 0.10 are considered an indication of fair fit, and RMSEA values above 0.10 indicated poor fit [29]. The following models were run: Model 1—overall sample (excluding CLI and FLI items which are specific to only the pediatric subgroups); Model 2—overall sample (replicating Model 1, but run with LI [five items]); Model 3—combined pediatric sample (including the caregiver reported CLI and FLI items); Model 4—combined pediatric sample (excluding the caregiver-reported CLI and FLI items and the CS items); and Model 5—combined pediatric sample (excluding the CLI and FLI items and the SS items).
3.2.2 Psychometric Score Performance
LIQ-GHD item-level frequencies and descriptives, as well as domain-level descriptives, were calculated for the overall, child, adolescent, and adult groups. Descriptive statistics included the mean, median, and range for each statistic calculated.
Item-to-item correlations were calculated for each item against the other items using Spearman’s rank order correlation coefficient (rho), with rho > 0.80 indicating potential redundancy between items.
Item-total correlations were examined between items and hypothesized domains, as well as items and other domain scores, following the multi-method multi-trait paradigm [30]. Items should correlate ≥ 0.40 with domains to which they are hypothesized to belong, and demonstrate no or weak correlation with other domains [31].
Internal consistency reliability was estimated using Cronbach’s coefficient alpha (α) [32], which ranges from 0 to 1, with α ≥ 0.70 being considered acceptable.
Intra-class correlation coefficients (ICCs) were calculated to assess test–retest reliability of the LIQ-GHD item and domain scores, using a two-way mixed model (Shrout and Fleiss ICC) [33]. The ICC ranges from 0.00 to 1.00 with a minimal acceptable level of 0.70 for group comparisons [34].
Construct-related validity was evaluated by generating convergent and discriminant validity correlation estimates and conducting a set of known-groups analyses.
For tests of convergent/discriminant validity, hypothesized relationships among LIQ-GHD and SIAQ variables were estimated using Spearman rho. Evidence for convergent validity was based on rho ≥ |0.40|, and evidence for discriminant validity was based on rho < |0.30| [30, 35].
Known-groups-methods analyses characterize the degree to which scores produced by a target questionnaire can distinguish among the groups hypothesized a priori to be clinically distinct [20]. Known groups were defined in five ways: group (i) changes to life routine in order to deal with GH injections; group (ii) number of missed injections; group (iii) satisfaction with overall experience taking GH treatment; group (iv) GHD severity; and group (v) overall health rating. These five groups were selected with the expectation that subgroup members would differ clinically and/or that they would have a higher score in the LIQ-GHD. We expected the LIQ-GHD scores to reflect the differences within these groups. Between-group differences were evaluated using the t test or analysis of variance, or non-parametric analyses such as the Mann–Whitney U test or Kruskal–Wallis test when normality assumptions were not met. For parametric analyses, standardized effect sizes (Cohen’s d, difference in means divided by pooled standard deviation [SD]) were calculated to quantify the magnitude of the differences between groups and interpreted as follows: d = 0.2 as a small effect size, d = 0.5 as a medium effect size, and d = 0.8 as a large effect size [36].
4 Results
4.1 Establishing Evidence of Content Validity
4.1.1 Targeted Review of Empirical Literature
A targeted search of empirical literature yielded 315 potentially relevant abstracts for review. Abstracts were screened and additional publications were identified from supplemental searches, resulting in a total of 30 publications selected for full review (see Fig. 2). Concepts emerging from the reviewed publications primarily focused on ease or difficulty of injection device use, injection regimen, injection-related adverse effects, and injection-related impacts. The identified concepts were organized into a literature-based conceptual model (see Fig. 3) and used to inform questionnaire modification and development.
4.1.2 Questionnaire Modification and Development
Two existing questionnaires, the IPAQ (a questionnaire measuring r-hGH device ease of use) and the BSW (a questionnaire measuring benefit from, satisfaction with, and willingness to continue treatment) were modified, and included in the draft LIQ-GHD, for this study’s context of use. New questionnaire content was developed to measure concepts identified during the literature review. Table 1 presents a summary of the LIQ-GHD item content and response option format.
4.1.3 Advice Meetings with Clinical Experts
Six clinical experts in the USA (n = 3) and the EU (n = 3) took part in advice meetings. All six experts had a minimum of 9 years’ experience treating individuals with GHD, and half had more than 20 years’ experience. During these meetings, experts were asked about their patients’ experience with r-hGH injections and how r-hGH injections impact the lives of patients and caregivers. Experts reported many of the same concepts identified in the literature (see Table 2). Experts were also asked to review the draft LIQ-GHD and provide feedback. Although individual experts had suggestions for revising the LIQ-GHD, there were no consistent or fundamental issues identified that would call into question the appropriateness and comprehensiveness of the questionnaire.
4.1.4 Questionnaire Revision
Following the expert advice meetings, the LIQ-GHD was organized into two versions: the LIQ-GHD-Adult (intended for adults ≥ 25 years of age) and the LIQ-GHD-Pediatric (intended for children and adolescents 3–17 years of age and their caregivers). The LIQ-GHD-Adult is a patient self-reported outcome (PRO) measure. For most questions, the LIQ-GHD-Pediatric is a COA intended for ‘dyad administration’ in which the patient and caregiver read and answer questions together. LIQ-GHD-Pediatric questions asking about the severity of injection symptoms are administered only with children and adolescents capable of reliable self-report, while some observer-reported outcome (ObsRO) items are answered by the caregiver about his/her own experiences, experiences of the family, and observable behaviors of the child.
4.1.5 Translatability Assessment
While some minor recommendations for formatting and word choice were made to increase the translatability of the instruments across languages, the translatability assessment confirmed that the LIQ-GHD-Adult and LIQ-GHD-Pediatric could be effectively translated into a wide range of languages.
4.1.6 Qualitative Interviews with Patients and Caregivers
Qualitative interviews were conducted with 21 individuals with GHD (11 children < 12 years of age and their caregivers, four adolescents 12–17 years of age and their caregivers, and six adults ≥ 25 years of age). Table 3 presents participant demographic and health information.
Concept elicitation results confirmed that the LIQ-GHD comprehensively captures key aspects of the r-hGH injection experience (see Table 2 for a summary of concepts reported by patients and caregivers). All injection-related SS concepts reported by > 25% of the total sample were included in the LIQ-GHD, as were all impact domains reported by patients and their caregivers.
Cognitive debriefing results indicated that the LIQ-GHD instructions, items, and response options were generally well-understood. While a few of the younger children had comprehension issues, the nature of the dyad administration mitigated this finding (i.e., in these instances, the caregiver read the question to the child and arrived at a response together with the child). Minor wording revisions were made following these interviews in an effort to improve patient understanding. In addition, questions were added to assess bleeding, bother caused by injections, and number of and reasons for missed injections.
4.2 Evaluation of Hypothesized Factor Structure and Score Psychometric Performance
A total of 224 participants were included in the online observational study, including 70 children (age 3–11 years)/caregiver dyads, 79 adolescent (age 12–17 years)/caregiver dyads, and 75 adults (age ≥ 25 years). The patient sample ranged from 3 to 87 years in age. More than half of the patients were male (58%), and the majority were white (84%) (see Table 3).
4.2.1 Confirmatory Factor Analysis
CFA using the overall sample demonstrated good-fit statistics for the hypothesized domain structure (without the family LI and caregiver LI items) using both the five- and seven-item patient LI domains (RMSEA = 0.07, CFI = 0.98 for each). CFA demonstrated good-fit statistics for both Model 1 (RMSEA = 0.07, CFI = 0.98) and Model 2 (RMSEA = 0.07, CFI = 0.98) in the overall group (with the travel and spending the night away from home [i.e., overnight] items correlated), supporting the hypothesized factor structure. Model 3 did not converge. CFA demonstrated good-fit statistics for Model 4 (five-item LI: RMSEA = 0.07, CFI = 0.97; seven-item LI: RMSEA = 0.08, CFI = 0.95) (with the travel and overnight items correlated). CFA demonstrated good-fit statistics for Model 5 (five-item LI: RMSEA = 0.07, CFI = 0.97; seven-item LI: RMSEA = 0.08, CFI = 0.96) (with the travel and overnight items correlated).
4.2.2 Item Distributions
The evaluation of item distributions for the full study sample demonstrated floor effects for most items of the LIQ-GHD, though no ceiling effects were observed for any items. For most items, all response options were used, although the upper end of the scale was not selected for some items. The findings for specific subanalyses based on patient age groups were similar to the overall sample. No missing data were observed as the electronic administration of the measure did not allow items to be skipped.
4.2.3 Item-to-Item Correlations
Evaluation of item-to-item correlations suggested possible redundancy between four sets of items in the LIQ-GHD: items assessing interference with spending the night away from home (i.e., overnight) and interference with travel, for patients, caregivers, and family members; items assessing interference with recreation and leisure activities and interference with social activities, for caregivers and family members; items assessing patient-reported and caregiver-reported bruising; and items assessing patient- and caregiver-reported bleeding.
4.2.4 Item-Total Correlations
In general, the items correlated as expected with their hypothesized domains in the overall, child, adolescent, and adult groups and, for the most part, items correlated more strongly with their hypothesized domains than with other domain scores. There were some exceptions to this; for example, while the bother item correlated well with its hypothesized seven-item LI domain, it correlated more strongly with the WtC and patient-reported symptoms (SS) domains. Item-total correlations for the WtC domain were generally moderate to weak (i.e., < 0.40 correlation). Not unexpectedly, moderate correlations were observed for patient LI and the caregiver LI and family LI items, and for the SS and caregiver-reported sign (CS) items.
4.2.5 Internal Consistency Reliability
For the overall study sample, internal consistency reliability was demonstrated for all domains (with Cronbach’s α ranging from 0.746 to 0.905), with two exceptions (see Table 4). Similarly, all domains in all subgroups met the threshold of α ≥ 0.70 for internal consistency reliability, with few exceptions (see Table 4).
4.2.6 Test–Retest Reliability
ICCs for the LIQ-GHD domain scores ranged from 0.761 to 0.918 for the overall sample (see Table 5). Results were similar when analyzed by subgroup, with an ICC > 0.70 being observed for most of the LIQ-GHD across age groups, with the exception of the EoIS and WtC in children (with ICC = 0.679 and ICC = 0.697, respectively).
4.2.7 Convergent and Divergent Validity
Table 6 presents correlations between LIQ-GHD and SIAQ domain scores for the overall group. As predicted, results demonstrated evidence of convergence between the PEoU and SIAQ Ease of use of the injection device scores, and between the WtC and SIAQ Satisfaction with self-injection scores. However, inconclusive results were found for the EoIS and SIAQ Satisfaction with self-injection scores and for the patient LI (five-item) and SIAQ satisfaction with self-injection scores. As predicted, inconclusive correlation results were found for caregiver LI (five-item) and SIAQ Feelings about injections scores, and divergence was found between family LI (five-item) and SIAQ Feelings about injection scores.
For the child group, evidence of convergence among domain scores was not found; however, evidence of divergence was found between caregiver LI (five-item) and SIAQ Feelings about injections scores and between family LI (five-item) and SIAQ Feelings about injections scores.
For the adolescent group, evidence of convergence was found for PEoU and SIAQ Ease of use of the injection device scores and for the WtC and SIAQ Satisfaction with self-injection scores, but not for other domains, as predicted; evidence of divergence was found for family LI (five-item) and SIAQ Feelings about injection scores.
For the adult group, evidence of convergence was found for all scores (PEoU and SIAQ Ease of use of the injection device, EoIS and SIAQ Satisfaction with self-injection domain, patient LI [five-item] and SIAQ Satisfaction with self-injection, and WtC and SIAQ Satisfaction with self-injection), as predicted.
4.2.8 Known Groups
For the overall sample, the LI (five-item) and EoIS domain scores differed significantly for groups reporting changes/no changes to life routine, satisfied/dissatisfied with treatment, and number of missed injections (see Table 7). Specifically, participants reporting frequent changes to life routine, dissatisfaction with treatment, and higher number of missed injections had higher LI and injection schedule difficulty (see Figs. 4, 5, 6 respectively). No significant differences were found based on self-rating of overall health or GHD severity level. Some similar results were observed in the child, adolescent, and adult group analyses; however, these analyses were limited due to small sample size.
5 Discussion
Results from this research, conducted in accordance with best practices [21, 22, 37, 38], provide evidence of the LIQ-GHD content and construct validity and score psychometric performance, based on a targeted literature review, expert advice meetings, interviews with patients and caregivers, and data from a quantitative field study.
The literature review revealed a paucity of data relating to r-hGH injection treatment burden (the most-cited references were related to diabetes [39,40,41,42,43]) and identified several functional health domains (e.g., emotional, role, travel, physical function) that may be impacted by daily injection treatment.
Results from the clinical expert advice meetings confirmed the literature findings and informed development of the draft LIQ-GHD.
Qualitative patient and caregiver interviews confirmed that the most important and relevant concepts related to r-hGH injection treatment burden were included in the LIQ-GHD, that participants understood and were able to answer the questions, and that it comprehensively covers relevant concepts. Interview results also confirmed the overall feasibility of the dyad administration approach.
Results from cognitive debriefing provide evidence that the minimum age for reliable self-report on symptom questions is 8 years. Thus, the administrative instructions for the LIQ-GHD specify that symptom questions be administered only to respondents ≥ 8 years of age, and questions that assess observable signs (e.g., bleeding and bruising questions) be administered to caregivers.
Following qualitative interviews, the LIQ-GHD was finalized and tested in a cross-sectional, observational field study. Results from the field study confirmed the hypothesized factor structure of the LIQ-GHD and yielded preliminary evidence of score reliability and construct validity in measuring treatment burden of daily r-hGH injections.
As observed in the literature review, evidence suggests that the burden of daily r-hGH injections has a negative effect on treatment adherence and thus on effectiveness. While data show that pediatric and adult patients with GHD may acclimate to daily r-hGH injections, they still continue to experience associated burden and impacts [44]. The availability of a less frequent injection schedule could address many of the burdens and barriers identified.
The research presented here adds to the r-hGH injection treatment burden evidence base and reinforces results from similar research undertaken by others that also identified daily injection LI, as well as emotional and social impacts [45, 46].
Evaluation of item frequency distributions identified a floor effect for many LIQ-GHD items, which is a possible study limitation and an important concern with regards to measurement. The floor effect was present but less notable for some questions (e.g., ‘spending the night away from home’ and ‘travel’ LI items, and the convenience question). Items with substantive floor or ceiling effects can limit the instrument’s range of measurement (and thereby limit the responsiveness of the tool). One possible strategy to mitigate the potential consequences of a floor effect is to extend the lowest extreme response anchor. However, for most of these items, the lowest response category is ‘Never’. One likely explanation for this finding is that most (if not all) patients in this target patient population follow a frequent (i.e., daily) r-hGh injection regimen, may have acclimated to that routine, and may not have any knowledge, exposure to, or experience with a less frequent regimen. Indeed, in this study’s qualitative interviews, patients and caregivers indicated that they have made adaptations to their lives to deal with the burden of daily treatment injections. This may explain the floor effects observed on the LIQ-GHD items. While this may be the case, establishing a standardized method for the assessment of LI is important for these patients. The LIQ-GHD includes items that are written in such a manner that they may be used (or easily modified) to assess the patient experience with varied injection regimens. Future studies employing the LIQ-GHD should be powered (e.g., ensuring sufficient sample size to detect an effect or change) with these considerations in mind.
Another possible limitation is the short interval between the time 1 and time 2 LIQ-GHD administrations for the evaluation of test–retest reliability (although efforts were made for the participants to complete other questionnaires during the interval). Additionally, some of the known-groups analyses could not be completed due to the small sample size for grouping categories. Because this was primarily a cross-sectional study design, longitudinal analyses were not conducted to evaluate score interpretation. Future psychometric research should replicate these reliability and validity analyses and evaluate score interpretation.
One other possible limitation to the data is that the development and testing of this LIQ-GHD was only carried out with US patients and caregivers. However, there does not appear to be evidence (e.g., from the literature) suggesting that the burden of daily r-hGH injections may differ substantially for patients residing in other parts of the world. Future research can confirm content relevance and performance of the LIQ-GHD in countries outside of the USA.
The benefits of a less frequent injection regimen might be further explored and elucidated in future research assessing the within-person difference in experience of more frequent (e.g., daily) versus less frequent (e.g., weekly) injections.
The LIQ-GHD may be useful for capturing and assessing aspects of the injection treatment burden of individuals receiving r-hGH injections. Addressing patient preferences for treatment may improve compliance, adherence, and, ultimately, clinical outcomes [47,48,49].
6 Conclusion
The LIQ-GHD is a new COA tool, designed for self- or dyad-administration, that has demonstrated evidence of content validity, reliability, and construct validity. The LIQ-GHD measures concepts that are important and relevant to patients (and their caregivers) and can be used to characterize the r-hGH injection treatment burden experienced by patients (and caregivers, where appropriate), to inform patient–healthcare provider communications and optimal individualized treatment decisions that may improve treatment compliance and adherence and long-term outcomes for GHD patients. The results of this research provide evidence that the LIQ-GHD is fit for use in clinical trials including adult patients (≥ 25 years of age), adolescents (12–17 years of age), and children (3–11 years of age) to establish treatment benefit in new GHD interventions.
References
Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah growth study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125(1):29–35.
Rona RJ, Tanner JM. Aetiology of idiopathic growth hormone deficiency in England and Wales. Arch Dis Child. 1977;52(3):197–208. https://doi.org/10.1136/adc.52.3.197.
Vimpani GV, Vimpani AF, Lidgard GP, Cameron EH, Farquhar JW. Prevalence of severe growth hormone deficiency. Br Med J. 1977;2(6084):427–30. https://doi.org/10.1136/bmj.2.6084.427.
Bao XL, Shi YF, Du YC, Liu R, Deng JY, Gao SM. Prevalence of growth hormone deficiency of children in Beijing. Chin Med J (Engl). 1992;105(5):401–5.
GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990–3. https://doi.org/10.1210/jcem.85.11.6984.
Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med. 1999;341(16):1206–16. https://doi.org/10.1056/NEJM199910143411607.
Melmed S, Kleinberg D. Anterior pituitary. In: Larsen P, Kronenberg H, Melmed S, Polonsky K, editors. Williams textbook of endocrinology. Philadelphia: WB Saunders Co.; 2003. p. 180–238.
Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Horm IGF Res. 2009;19(1):1–11. https://doi.org/10.1016/j.ghir.2008.08.001.
Bullinger M, Quitmann J, Power M, Herdman M, Mimoun E, DeBusk K, et al. Assessing the quality of life of health-referred children and adolescents with short stature: development and psychometric testing of the QoLISSY instrument. Health Qual Life Outcomes. 2013;11:76. https://doi.org/10.1186/1477-7525-11-76.
Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143–54. https://doi.org/10.4158/ep.14.2.143.
Cutfield WS, Derraik JG, Gunn AJ, Reid K, Delany T, Robinson E, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6(1):e16223. https://doi.org/10.1371/journal.pone.0016223.
Bozzola M, Pagani S, Iughetti L, Maffeis C, Bozzola E, Meazza C. Adherence to growth hormone therapy: a practical approach. Horm Res Paediatr. 2014;81(5):331–5. https://doi.org/10.1159/000357975.
Koledova E, Stoyanov G, Ovbude L, Davies PSW. Adherence and long-term growth outcomes: results from the easypod. Endocr Connect. 2018;7(8):914–23. https://doi.org/10.1530/EC-18-0172.
Elliott RA, Marriott JL. Standardised assessment of patients’ capacity to manage medications: a systematic review of published instruments. BMC Geriatr. 2009;9:27. https://doi.org/10.1186/1471-2318-9-27.
Berhe DF, Taxis K, Haaijer-Ruskamp FM, Mulugeta A, Mengistu YT, Burgerhof JGM, et al. Impact of adverse drug events and treatment satisfaction on patient adherence with antihypertensive medication—a study in ambulatory patients. Br J Clin Pharmacol. 2017;83(9):2107–17. https://doi.org/10.1111/bcp.13312.
Ngoh LN. Health literacy: a barrier to pharmacist-patient communication and medication adherence. J Am Pharm Assoc (2003). 2009;49(5):132–46. https://doi.org/10.1331/JAPhA.2009.07075(quiz e47–9).
Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79(4):189–96. https://doi.org/10.1159/000350251.
Pleil AM, Kimel M, McCormack J, Rajicic N, Hey-Hadavi J. Psychometric assessment of the Injection Pen Assessment Questionnaire (IPAQ): measuring ease of use and preference with injection pens for human growth hormone. Health Qual Life Outcomes. 2012;10:126. https://doi.org/10.1186/1477-7525-10-126.
Ungar WJ, Mirabelli C, Cousins M, Boydell KM. A qualitative analysis of a dyad approach to health-related quality of life measurement in children with asthma. Soc Sci Med. 2006;63(9):2354–66.
US Food and Drug Administration. Guidance for industry on patient-reported outcome measures: use in medical product development to support labeling claims; availability. Fed Reg. 2009;74(235):65132–3.
European Medicines Agency. Reflection paper on the Regulatory Guidance for the Use of Health-related Quality of Life (HRQL) measures in the evaluation of medicinal products. EMEA/CHMP/EWP/139391/2004. London: European Medicines Agency; 2005.
Matza LS, Patrick DL, Riley AW, Alexander JJ, Rajmil L, Pleil AM, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO Good Research Practices for the Assessment of Children and Adolescents Task Force. Value Health. 2013;16(4):461–79. https://doi.org/10.1016/j.jval.2013.04.004.
Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65.
Pleil AM, Coyne KS, Reese PR, Jumadilova Z, Rovner ES, Kelleher CJ. The validation of patient-rated global assessments of treatment benefit, satisfaction, and willingness to continue—the BSW. Value Health. 2005;8(Suppl 1):S25–34. https://doi.org/10.1111/j.1524-4733.2005.00069.x.
Hauffa BP, Touraine P, Urquhart-Kelly T, Koledova E. Managing transition in patients treated with growth hormone. Front Endocrinol. 2017;8:346. https://doi.org/10.3389/fendo.2017.00346.
Clayton PE, Cuneo RC, Juul A, Monson JP, Shalet SM, Tauber M. Consensus statement on the management of the GH-treated adolescent in the transition to adult care. Eur J Endocrinol. 2005;152(2):165–70. https://doi.org/10.1530/eje.1.01829.
Cook DM, Yuen KC, Biller BM, Kemp SF, Vance ML. American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients—2009 update. Endocr Pract. 2009;15(Suppl 2):1–29. https://doi.org/10.4158/ep.15.s2.1.
Keininger D, Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ). Health Qual Life Outcomes. 2011;9:2. https://doi.org/10.1186/1477-7525-9-2.
Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6(1):1–55.
Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol Bull. 1959;56(2):81–105.
Nunnally J, Bernstein I. Psychometric theory. 3rd ed. New York: McGraw-Hill; 1994.
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8.
Lohr KN, Aaronson NK, Alonso J, Burnam MA, Patrick DL, Perrin EB, et al. Evaluating quality-of-life and health status instruments: development of scientific review criteria. Clin Ther. 1996;18(5):979–92.
Cappelleri JC, Zou KH, Bushmakin AG, Alvir JMJ, Alemayehu D, Symonds T. Patient-reported outcomes: measurement, implementation and interpretation. Boca Raton: CRC Press; 2013.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Earlbaum Associates; 1988.
Lasch KE, Marquis P, Vigneux M, Abetz L, Arnould B, Bayliss M, et al. PRO development: rigorous qualitative research as the crucial foundation. Qual Life Res. 2010;19(8):1087–96.
Leidy NK, Vernon M. Perspectives on patient-reported outcomes: content validity and qualitative research in a changing clinical trial environment. Pharmacoeconomics. 2008;26(5):363–70.
Kruger DF, LaRue S, Estepa P. Recognition of and steps to mitigate anxiety and fear of pain in injectable diabetes treatment. Diabetes Metab Syndr Obes. 2015;8:49–56. https://doi.org/10.2147/dmso.s71923.
Hauber AB, Johnson FR, Sauriol L, Lescrauwaet B. Risking health to avoid injections: preferences of Canadians with type 2 diabetes. Diabetes Care. 2005;28(9):2243–5.
Hauber AB, Nguyen HT, Posner J, Ervin C, LaRue S, Kalsekar I. Patient preferences for frequency of glucagon-like peptide-1 receptor agonist (GLP-1RA) injections in the treatment of type 2 diabetes [abstract]. ISPOR 19th Annual International Meeting; 31 May–4 Jun 2014; Montreal.
Hauber AB, Tunceli K, Yang JC, Gantz I, Brodovicz KG, Alexander CM, et al. A survey of patient preferences for oral antihyperglycemic therapy in patients with type 2 diabetes mellitus. Diabetes Ther. 2015;6(1):75–84. https://doi.org/10.1007/s13300-015-0094-2.
Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–30. https://doi.org/10.1007/s10198-010-0224-8.
Brod M, Hojbjerre L, Wilkinson L, Alolga SL, Rasmussen MH. Assessing the treatment burden for growth hormone deficiency (GHD) in children: concept elicitation results supporting the development of the Treatment Burden Measure for Childhood GHD (Tb-Cghd) [abstract]. Value Health. 2015;18(7):A676. https://doi.org/10.1016/j.jval.2015.09.1999.
Brod M, Højbjerre L, Wilkinson L, Alolga SL, Rasmussen MH. Assessing the impact of growth hormone deficiency (GHD) in children: concept elicitation results supporting the development of the Treatment-Related Impact Measure for Childhood Ghd (TRIM-CGHD) [abstract]. Value Health. 2015;18(7):A674. https://doi.org/10.1016/j.jval.2015.09.1990.
Brod M, Højbjerre L, Alolga SL, Beck JF, Wilkinson L, Rasmussen MH. Understanding treatment burden for children treated for growth hormone deficiency. Patient. 2017;10(5):653–66. https://doi.org/10.1007/s40271-017-0237-9.
Hauber AB, Nguyen H, Posner J, Kalsekar I, Ruggles J. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes. Curr Med Res Opin. 2016;32(2):251–62. https://doi.org/10.1185/03007995.2015.1117433.
Reginster JY, Rabenda V. Patient preference in the management of postmenopausal osteoporosis with bisphosphonates. Clin Interv Aging. 2006;1(4):415–23.
Mansfield C, Sikirica MV, Pugh A, Poulos CM, Unmuessig V, Morano R, et al. Patient preferences for attributes of type 2 diabetes mellitus medications in Germany and Spain: an online discrete-choice experiment survey. Diabetes Ther. 2017;8(6):1365–78. https://doi.org/10.1007/s13300-017-0326-8.
Acknowledgements
Components of this research were summarized in individual poster presentations, including: ISPOR 22nd Annual International Meeting, Boston, MA, USA 20–24 May 2017; ISPOR 20th Annual European Congress, Glasgow, Scotland, 4–8 November 2017; ISPOR 23rd Annual International Meeting, Baltimore, MD, USA, 19–23 May 2018; and the 57th Annual European Society for Paediatric Endocrinology (ESPE) Meeting, Athens, Greece, 27–29 September 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DMT-B, AY, REL, MK, EL, and AS are employed by Adelphi Values. JL and AP are employed by Pfizer. AMP is an independent research and evaluation consultant who was employed by Pfizer at the time of the research.
Sponsorship/funding statement
This study was sponsored by Pfizer. Diane M. Turner-Bowker, Andrew Yaworsky, Roger E. Lamoureux, Masami Kelly, Emily Love, and Alan Shields are employees of Adelphi, who were paid consultants by Pfizer in connection with the development of this manuscript.
Author contributions
DMT-B, AY, AP, AMP, AS, and JL contributed to the design of the study. DMT-B, AY, AP, RL, MK, EL, and JL were involved in the acquisition and analysis of data for the work. All authors were involved in the interpretation of the results. DMT-B, AY, RL, and JL drafted the manuscript. All authors read, reviewed, and approved the final manuscript.
Data Availability Statement
The data that support the findings of this study are available from Pfizer, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of Pfizer. The authors can confirm that relevant data are included in the article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Turner-Bowker, D.M., Yaworsky, A., Palladino, A. et al. Development and Psychometric Evaluation of the Life Interference Questionnaire for Growth Hormone Deficiency (LIQ-GHD) to Assess Growth Hormone Injection Burden in Children and Adults. Patient 13, 289–306 (2020). https://doi.org/10.1007/s40271-019-00405-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-019-00405-7