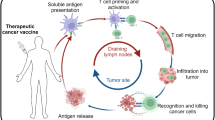
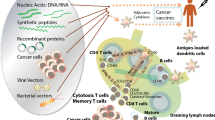
Abstract
The promise of immune-based therapies to treat cancer has been realized over the last several years with several breakthrough therapies, including T-cell checkpoint inhibitors and chimeric antigen receptor (CAR)-T cell therapies. While cancer vaccines have been investigated for many decades, to date only one has been approved in the USA as a treatment for existing cancer. The failure of several anti-tumor vaccines in large phase III trials has led many to question their future role in cancer treatment. Trials to date have demonstrated that many cancer vaccines can elicit tumor-specific T cells, but these T cells may be insufficient to mediate substantial anti-tumor effects without concurrent blockade of tumor-resistance mechanisms. Emerging data from preclinical models and clinical trials demonstrate that cancer vaccines have greater activity in low-volume disease and in combination with other immune-modulating therapies, including T-cell checkpoint blockade, targeting these resistance mechanisms. Because T-cell checkpoint therapies likely require the presence or activity of tumor-specific T cells, cancer vaccines may be optimal agents to use in combination to enable these therapies to work for greater numbers of patients. Future trials will explore optimal vaccine approaches and antigens that work best in combination treatment approaches and in earlier stages of disease.
Similar content being viewed by others
Cancer vaccines have demonstrated efficacy in eliciting anti-tumor responses in preclinical models, and safety and immune responses to intended target antigens in clinical trials. |
Preclinical studies and emerging clinical data suggest that the efficacy of cancer vaccines will likely be greater, and applicable to many cancer types, when used in combination with treatments targeting mechanisms of tumor immune evasion. |
1 Introduction
For over a century, there has been interest in using the immune system to target malignant cells as a treatment for cancer. That long history has been punctuated with some evidence of activity, leading to the approval of specific cytokines [e.g., interferon (IFN)-α and interleukin-2] for the treatment of melanoma and renal cell cancer and of non-specific immune-modulating therapies [e.g., Bacillus Calmette-Guérin (BCG)] for the treatment of superficial bladder cancer. However, efforts to generate clinically effective tumor-specific immunity by means of vaccination have largely been unsuccessful, despite evidence of anti-tumor activity in preclinical models. Over the last several years, there have been great strides in the field of cancer immunotherapy, largely because of a greater understanding of T-cell signaling and regulation. In particular, the use of T-cell checkpoint inhibitors [anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), anti-programmed cell death protein 1 (PD-1), and/or anti-programmed death-ligand 1 (PD-L1)] has revolutionized the care of patients with melanoma, lung cancer, renal cell cancer, or bladder cancer, among others [1,2,3,4]. In 2010, the first anti-tumor vaccine, sipuleucel-T, was approved for the treatment of advanced prostate cancer [5]. These successes led cancer immunotherapy to be deemed the scientific breakthrough of the year in 2013 [6]. In 2015, an oncolytic herpes virus, talimogene laherparepvec, delivered as an in situ immunotherapy, was approved for the treatment of melanoma [7]. Within the last 2 months of this writing in 2017, the first immunotherapeutic gene therapy, using autologous T cells engineered to express chimeric antigen receptor (CAR)-T cells recognizing cluster of differentiation (CD)-19, was approved as a treatment for B-cell malignancies, on the basis of clinical trials demonstrating dramatic and durable eradication of disease [8]. Together, all these advances have furthered enthusiasm in the field to develop other immune-based therapies and apply them in combination with other cancer therapies. The current article focuses on the potential role of anti-tumor vaccines in this quickly developing armamentarium of novel cancer immunotherapies.
2 Overview
The concept of anti-tumor vaccination gained enthusiasm nearly 100 years ago following successes with anti-viral vaccines. Specifically, given the findings that delivery of inactivated viruses could protect individuals from subsequent challenge with live virus, many early efforts attempted to treat patients with inactivated autologous or allogeneic tumor cells to generate tumor-specific immunity. These early attempts did not have much success, and hence later attempts focused on different adjuvants and means to increase the immunogenicity of tumor cells. For example, Dranoff et al. [9] demonstrated that engineering tumor cells to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) enabled them to confer better protective immunity to subsequent tumor challenge. This approach has been evaluated as a treatment approach for many different types of cancer, and while early trials demonstrated evidence of clinical activity, randomized phase III trials did not meet endpoints demonstrating superiority over other treatments when used as a single agent [10, 11].
2.1 Choice of Target
Whole-cell vaccine approaches such as those described in the previous sections have an advantage of being agnostic about the specific target of the immune response, permitting the host to “choose” a relevant antigenic target. However, a theoretical disadvantage is that an immune response elicited with vaccination may be ineffective (targeting an irrelevant antigen) or that a potentially therapeutic immune response may be diluted in the context of concurrent immunization with many other irrelevant antigens. Investigators studying anti-microbial vaccines identified that immunity to specific microbial antigens could confer protective immunity. For example, immunity to the hepatitis B surface antigen (HBsAg) was most associated with protection and resistance to re-infection by hepatitis B [12]. This led to the development of recombinant vaccines specifically targeting HBsAg, an approach that simplified vaccine development and enabled evaluation of antigen-specific immunity as a measure of vaccine efficacy. Coincidentally, protection from hepatitis B by vaccination has led to a worldwide decrease in the incidence of hepatocellular carcinoma [13]. Similarly, targeting human papillomavirus (HPV) by vaccination has led to worldwide decreases in cervical cancer and likely other HPV-driven tumors [14, 15]. For several decades, this concept drove the tumor vaccine field to identify optimal tumor-associated antigens and to begin prioritizing tumor antigens as targets for vaccines to treat human cancers [16]. In addition, the realization from preclinical models that effective anti-tumor immunity was more dependent on T-cell immunity than humoral immunity suggested that vaccination methods needed to demonstrate antigen-specific T-cell immunity. Consequently, efforts to identify preferred immunization approaches, with specific tumor-associated antigens, have dominated efforts in preclinical studies and human trials over the last 20 years. Some early studies have suggested anti-tumor effects and have led to large randomized trials. One vaccine, sipuleucel-T, an autologous antigen-presenting cell vaccine loaded ex vivo with a prostate-associated antigen, prostatic acid phosphatase (PAP), did demonstrate improved overall survival in a randomized, placebo-controlled phase III trial, leading to its approval by the US FDA in 2010 [5]. To date, this is the only vaccine approved in the USA as a treatment for existing cancer, and it continues to be used to treat patients with advanced prostate cancer. Of note, patients with the greatest survival benefit did have evidence of immune response to the target antigen, consistent with the proposed mechanism of action [17]. In addition, retrospective studies have suggested that patients with the greatest degree of benefit were those with lower-volume disease [18]. Many other cancer vaccines, predominantly peptide-based vaccines, have failed to demonstrate clinical anti-tumor efficacy in phase III trials despite evidence of immunological activity (Table 1). These observations have led many to question whether vaccines will be “successful” as anti-tumor therapies.
2.2 Increasing Immunogenicity
Early failures of anti-tumor vaccines in clinical trials, in many cases with little evidence of systemic immunity elicited to the target antigen, led many investigators to seek to improve the immunogenicity of vaccines, assuming a higher magnitude of immune cells elicited should confer a better outcome. However, several clinical trials, despite demonstrating evidence of immunity, failed to demonstrate substantial anti-tumor efficacy. Moreover, studies of adoptive immunotherapy, in which high numbers of antigen-specific T cells with demonstrable cytolytic activity could be infused, have similarly demonstrated little anti-tumor efficacy in the absence of pre-conditioning regimens that might deplete the host of immune-suppressive mechanisms [19]. Collectively, these findings suggest that anti-tumor vaccines can elicit anti-tumor responses and memory immune responses, and the limitation is not one of eliciting sufficient numbers of the “right kind” of cells, rather that these cells can be inactivated in tumor-bearing hosts. Such findings suggest that anti-tumor vaccines, when used in the context of patients with existing tumors, are unlikely to succeed without accounting for mechanisms of resistance within tumors to avoid immune detection and destruction.
2.3 Lessons from Prior Successes
Notwithstanding, anti-tumor vaccines have demonstrated activity in multiple immune-competent animal models. Anti-microbial vaccines have clear efficacy, in some cases leading to the eradication of human disease by preventing infection and spread. So, what can be learned from these successes that could be applied to human cancer vaccines? Clearly, the greatest difference is that anti-microbial vaccines are not used to treat existing infections but rather to generate protective immunity to prevent subsequent challenge. In contrast, with few exceptions, anti-tumor vaccines have been evaluated in patients with existing disease and, in most cases, in patients with large tumor burdens. Most preclinical models have suggested that, while vaccines can protect from tumor challenge, they are less effective in treating established tumors [20]. This is perhaps obvious because a hallmark of cancer is the development of mechanisms of evading immune detection, including decreased expression of major histocompatibility complex (MHC) class I, expression of immunosuppressive cytokines, and infiltration by immune regulatory/suppressive cell populations [21]. Thus, using vaccines in combination with efforts to target these mechanisms of resistance would seem logical [22]. When tumor vaccines were evaluated in murine models with established tumors, anti-tumor effects were generally greater when vaccines were initiated with small tumor volumes rather than with large volumes [23, 24]. Fewer clinical trials have been performed in adjuvant settings or settings of minimal residual disease, which would be predicted to be the settings in which vaccines could potentially have anti-tumor activity for established tumors. In addition, with the exception of vaccines targeting mucin 1 (MUC1), few studies have been conducted in the preventive setting, a setting in which vaccines might be most expected to have single-agent activity [25].
What can be learned from the successes of other immunotherapy approaches that could be applied to human cancer vaccines? Over the last 5 years, the most dramatic anti-tumor responses have been observed with T-cell checkpoint inhibitors, notably agents targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein-1 (PD-1)/programmed death-ligand 1 (PD-L1), and CAR-T cell approaches targeting CD19. These agents interfere with or supplant normal T-cell signaling and regulation, suggesting this may be important to the efficacy of vaccine-induced T cells. CTLA-4 blockade timed with vaccination would be predicted to augment the number and proliferation of elicited T cells, and specifically target regulatory T cells expressing CTLA-4. In fact, murine studies in a transgenic prostate tumor model demonstrated that neither a GM-CSF-expressing vaccine nor CTLA-4 blockade had substantial anti-tumor activity unless used together [26]. These findings were confirmed in human clinical trials for prostate cancer in which GM-CSF-secreting vaccines or CTLA-4 blockade did not demonstrate significant anti-tumor activity when used alone [27, 28] but had more substantial activity when used in combination [29]. In the case of PD-1/PD-L1 blockade, these agents presumably require T cells responsive to tumors that express PD-1 to mediate anti-tumor effects. Thus, therapies that can augment tumor-reactive PD-1-expressing CD8+ T cells, including tumor vaccines, would be predicted to improve the efficacy of PD-1/PD-L1 blockade. In fact, in the case of prostate cancer, a disease for which an anti-tumor vaccine has been approved and for which there has been less evidence of single-agent activity of T-cell checkpoint inhibitors, it is perhaps noteworthy that these cancers typically do not have increased numbers of infiltrating T cells relative to tumors such as melanomas [30]. Consequently, vaccine therapies aimed at increasing the number of tumor-specific T cells, in combination with therapies that can increase their ability to infiltrate tumors and lyse tumor cells, should theoretically be of even greater benefit. Moreover, it is known that PD-1 expression increases with T-cell activation induced by vaccination [31]. We have demonstrated that PD-1 blockade at the time of T-cell activation with vaccines can mediate more substantial anti-tumor efficacy in murine models and in patients with advanced, metastatic prostate cancer in an ongoing clinical trial [32,33,34].
3 Components of Combination Immunotherapy
So how do we integrate this information and what is the future for anti-tumor vaccines? First, anti-tumor vaccines obviously do “work” in that they can clearly elicit or augment immunity to the intended tumor-associated target antigen(s). The overwhelming data from clinical trials to date indicate they are safe and cause few adverse effects. However, vaccines alone have generally not been able to overcome the mechanisms of resistance present within tumors to avoid detection and destruction mediated by vaccine-induced immune cells. Thus, it seems clear that these therapies, if not used in a setting of low or absent tumor volume, will need to be used in combination, as has been suggested by others [22, 35]. Murine studies and early clinical studies have already demonstrated this, and a large part of future research will be to determine optimal agents and sequencing of these agents. It should be highlighted that, despite the revolutionary impact of T-cell checkpoint inhibitor therapies, these therapies still only work as monotherapies for a minority of patients. Having existing tumor-specific T cells, and CD8+ T cells in particular, is likely critical to the success of these therapies, at least for PD-1/PD-L1 blockade [36]. Hence, it seems logical that agents that can increase the number of tumor-reactive CD8+ T cells should be preferred agents to use in combination with T-cell checkpoint therapies, potentially increasing the number of patients who could benefit from these therapies. While certainly chemotherapy, radiation therapy, hormonal therapy, and some small-molecule-targeted therapies might activate CD8+ T cells and are being evaluated in combination with T-cell checkpoint inhibitors, the ability of vaccines to elicit or augment only tumor-specific CD8+ T cells should be preferable. That is, in addition to the safety of vaccines compared with some of these other therapies, targeting tumor-specific T cells should minimize toxicity and increase the likelihood of activating T cells reactive only to tumor. The use of other agents to disrupt physical or vascular barriers, or other immunosuppressive cell populations, within the tumor microenvironment may also be necessary to optimally treat established tumors in combination with vaccines. Second, if cancer vaccines are to be evaluated as single agents, this should be in settings of low or absent tumor volume. This contrasts with the general approach of evaluating new cancer therapies for single-agent activity in patients with advanced disease prior to considering combination therapies. Given the safety of vaccines observed to date, it is our opinion that they can be reasonably evaluated in the adjuvant or minimal disease setting. Moreover, single-agent studies in these settings permit an evaluation of biological/immune effect over a longer period of time, as we have previously demonstrated, to identify appropriate treatment schedules [37, 38]. Finally, the optimal vaccine approach(es) and target antigen(s) remains unknown and will continue to be evaluated in clinical trials. The identification of tumor-specific mutation-associated neo-epitopes as possible vaccine antigens attracts great enthusiasm, based on preclinical studies demonstrating that these are frequently the epitopes recognized by CD8+ T cells “unleashed” by PD-1 blockade [39]. However, whether targeting these epitopes is in fact superior to other non-mutated, shared antigens if similarly delivered in combination with T-cell checkpoint inhibitors or other immune-modulating therapies remains unknown. This is a critically important question because the most common solid tumors do not have high mutation burdens and may not have identifiable mutation-associated neo-epitopes. Recently, two clinical trials using vaccine approaches targeting mutation-associated neo-epitopes were reported [40, 41]. In both trials, some patients experienced objective responses, but these individual patients also received T-cell checkpoint inhibitors. Since objective responses have also been observed in patients treated with vaccines targeting shared antigens in combination with T-cell checkpoint blockade [29, 42], it remains important to determine whether there is an advantage to specifically targeting mutation-associated neo-epitopes. That is, if targeting these neo-epitopes by vaccination with T-cell checkpoint blockade is no better than targeting an “off-the-shelf” antigen with the same T-cell checkpoint blockade, then it may not be reasonable to employ this higher-cost, individualized vaccine therapy.
4 Summary
Cancer vaccines have demonstrated activity in preclinical models, and have been safe and immunologically active, but they have demonstrated only modest anti-tumor activity when employed as single agents in clinical trials. While one anti-tumor vaccine has been approved as a therapy for advanced-stage prostate cancer, most have not demonstrated superior activity in randomized phase III trials. Data from preclinical models have demonstrated that they have their greatest effect in settings of low or absent tumor volume, suggesting that their best likelihood of success as monotherapies will be as prophylactic treatments or in adjuvant or minimal residual disease settings. Emerging data from preclinical studies and early clinical trials demonstrate that anti-tumor vaccines can treat established tumors when used in combination with agents targeting tumor mechanisms of resistance. Hence, cancer vaccines for the treatment of established tumors will be best used in combination with T-cell checkpoint inhibitors and/or other immune- and tumor microenvironment-modulating therapies. In fact, cancer vaccines may be required to increase the number of patients who could benefit from T-cell checkpoint inhibitor therapies. Over the next several years, clinical trials will continue to explore optimal vaccine approaches and target antigens for use in patients with earlier stages of disease and in combination treatments.
References
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Ning YM, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, Palmby T, Fu W, Liu Q, Goldberg KB, Kim G, Pazdur R. FDA approval summary: atezolizumab for the treatment of patients with progressive advanced urothelial carcinoma after platinum-containing chemotherapy. Oncol. 2017;22(6):743–9.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I. Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22.
Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–3.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8.
Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: promise and complexities of a $475000 cancer drug. JAMA. 2017;318(19):1861–2.
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90(8):3539–43.
Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, Jablons D, Aimi J, Lin A, Hege K. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13(6):555–62.
Ward JE, McNeel DG. GVAX: an allogeneic, whole-cell, GM-CSF-secreting cellular immunotherapy for the treatment of prostate cancer. Expert Opin Biol Ther. 2007;7(12):1893–902.
Krugman S. Viral hepatitis type B: propects for active immunization. Dev Biol Stand. 1975;30:363–7.
Tsai HJ. Clinical cancer chemoprevention: From the hepatitis B virus (HBV) vaccine to the human papillomavirus (HPV) vaccine. Taiwan J Obstet Gynecol. 2015;54(2):112–5.
Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, Goldie SJ, Harper DM, Kinney W, Moscicki AB, Noller KL, Wheeler CM, Ades T, Andrews KS, Doroshenk MK, Kahn KG, Schmidt C, Shafey O, Smith RA, Partridge EE, Gynecologic Cancer Advisory G, Garcia F. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57(1):7–28.
Osazuwa-Peters N. Human papillomavirus (HPV), HPV-associated oropharyngeal cancer, and HPV vaccine in the United States—do we need a broader vaccine policy? Vaccine. 2013;31(47):5500–5.
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–37.
Sheikh NA, Petrylak D, Kantoff PW, Dela Rosa C, Stewart FP, Kuan LY, Whitmore JB, Trager JB, Poehlein CH, Frohlich MW, Urdal DL. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–47.
Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, Kantoff PW. Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297–302.
Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99(25):16168–73.
Nanni P, Nicoletti G, Palladini A, Croci S, Murgo A, Antognoli A, Landuzzi L, Fabbi M, Ferrini S, Musiani P, Iezzi M, De Giovanni C, Lollini PL. Antimetastatic activity of a preventive cancer vaccine. Cancer Res. 2007;67(22):11037–44.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219–33.
Cavallo F, Di Carlo E, Butera M, Verrua R, Colombo MP, Musiani P, Forni G. Immune events associated with the cure of established tumors and spontaneous metastases by local and systemic interleukin 12. Cancer Res. 1999;59(2):414–21.
Allione A, Consalvo M, Nanni P, Lollini PL, Cavallo F, Giovarelli M, Forni M, Gulino A, Colombo MP, Dellabona P, et al. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994;54(23):6022–6.
Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res. 2013;6(1):18–26.
Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–8.
Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24(5):419–24.
Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, Piulats JM, Gonzalez Mella P, Ng SS, Jaeger D, Parnis FX, Franke FA, Puente J, Carvajal R, Sengelov L, McHenry MB, Varma A, van den Eertwegh AJ, Gerritsen W. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35(1):40–7.
van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, Pinedo HM, Scheper RJ, Stam AG, von Blomberg BM, de Gruijl TD, Hege K, Sacks N, Gerritsen WR. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):509–17.
Fujii T, Shimada K, Asai O, Tanaka N, Fujimoto K, Hirao K, Konishi N. Immunohistochemical analysis of inflammatory cells in benign and precancerous lesions and carcinoma of the prostate. Pathobiol J Immunopathol Mol Cell Biol. 2013;80(3):119–26.
Hokey DA, Johnson FB, Smith J, Weber JL, Yan J, Hirao L, Boyer JD, Lewis MG, Makedonas G, Betts MR, Weiner DB. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur J Immunol. 2008;38(5):1435–45.
Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 epitope-modified DNA vaccine immunization. Cancer Immunol Res. 2015;3(8):946–55.
Zahm CD, Colluru VT, McNeel DG. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T cells. Cancer Immunol Res. 2017;5(8):630–41.
McNeel DG, Eickhoff J, Jeraj R, Staab MJ, Straus J, Rekoske BT, Liu G. DNA vaccine with pembrolizumab elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). In: 2016 National meeting of the Socitey for Immunotherapy of Cancer. 2016. (abstract 369)
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–51.
Danilova L, Wang H, Sunshine J, Kaunitz GJ, Cottrell TR, Xu H, Esandrio J, Anders RA, Cope L, Pardoll DM, Drake CG, Taube JM. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci USA. 2016;113(48):E7769–77.
McNeel DG, Dunphy EJ, Davies JG, Frye TP, Johnson LE, Staab MJ, Horvath DL, Straus J, Alberti D, Marnocha R, Liu G, Eickhoff JC, Wilding G. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J Clin Oncol. 2009;27(25):4047–54.
McNeel DG, Becker JT, Eickhoff JC, Johnson LE, Bradley E, Pohlkamp I, Staab MJ, Liu G, Wilding G, Olson BM. Real-time immune monitoring to guide plasmid DNA vaccination schedule targeting prostatic acid phosphatase in patients with castration-resistant prostate cancer. Clin Cancer Res. 2014;20(14):3692–704.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, Mulder GE, Toebes M, Vesely MD, Lam SS, Korman AJ, Allison JP, Freeman GJ, Sharpe AH, Pearce EL, Schumacher TN, Aebersold R, Rammensee HG, Melief CJ, Mardis ER, Gillanders WE, Artyomov MN, Schreiber RD. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–81.
Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–21.
Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, Bukur V, Tadmor AD, Luxemburger U, Schrors B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Muller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Bruck AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Holler C, Utikal J, Huber C, Loquai C, Tureci O. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–6.
McNeel DG, Eickhoff J, Jeraj R, Staab MJ, Straus J, Scarpelli M, Wargowski E, Liu G. DNA vaccine with pembrolizumab elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). In: 2017 ASCO-SITC Clinical Immuno-Oncology Symposium (Orlando, FL). 2017. (abstract 168)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
DGM was supported in this work by National Institutes of Health (NIH)/National Cancer Institute (NCI) (R01 CA219154).
Conflict of interest
DGM has ownership interest in, has received research support from, and serves as consultant to Madison Vaccines, Inc., which has licensed intellectual property related to this content.
Rights and permissions
About this article
Cite this article
McNeel, D.G. Therapeutic Cancer Vaccines: How Much Closer Are We?. BioDrugs 32, 1–7 (2018). https://doi.org/10.1007/s40259-017-0257-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-017-0257-y