Abstract
Background
Cost-utility analysis (CUA) is widely used for health technology assessment; however, concerns exist that cost-utility analysts may suggest higher cost-effectiveness thresholds (CETs) to compensate for technologies of relatively lower value.
Objective
We explored whether selection of a CUA study’s CET was endogenous to estimated incremental cost-effectiveness ratios (ICERs).
Methods
We systematically reviewed the US cost-effectiveness literature between 2000 and 2017 where studies with explicit CET and ICERs were included. We classified the ratio of studies hypothesized to analyze cost-effective technologies at low CETs (i.e., less than $100,000/quality-adjusted life-year [QALY]) vs higher CETs (i.e., $100,000–$150,000/QALY) relative to their ICER, using a Chi square test to examine whether technologies that were cost effective at high CETs would still be cost effective at lower thresholds. We also performed fixed-effects linear regression exploring the associations between ICERs and reported CETs over time.
Results
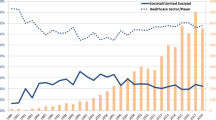
Among 317 ICERs reviewed: (A) 185 had an ICER < $50,000/QALY; (B) 53 had $50,000 ≤ ICER, < $100,000; (C) 20 had $100,000 ≤ ICER < $150,000; and (D) 59 had an ICER ≥ $150,000. Chi square testing showed a strong association (p < 0.001) between estimated ICER values and chosen CET, illustrating a lack of independence between the two. The regression analysis indicated that CETs have a baseline value of $52,000 and grow by $0.37 for each dollar increase in the estimated ICER.
Conclusions
Cost-effectiveness thresholds represent the hypothesis tests of typical CUAs. Our analysis highlights that most CUAs that cite high CETs also result in greater ICERs for the novel interventions that they investigate; thus, these interventions would otherwise not have been cost effective at lower CETs. Selection of a CET may come after the ICER is calculated to infer value that suits a hypothesis.

Similar content being viewed by others
References
Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA. 2016;316(8):858–71.
Pandya A. Adding cost-effectiveness to define low-value care. JAMA. 2018;319(19):1977–8.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103.
Neumann PJ, Kim DD, Trikalinos TA, Sculpher MJ, Salomon JA, Prosser LA, et al. Future directions for cost-effectiveness analyses in health and medicine. Med Decis Mak. 2018;38(7):767–77.
Sculpher M, Claxton K, Pearson SD. Developing a value framework: the need to reflect the opportunity costs of funding decisions. Value Health. 2017;20(2):234–9.
Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19(8):929–35.
Gold M, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
Neumann PJ, Sanders GD, Russel LB, Siegel JE, Ganiats TG, editors. Cost-effectiveness in health and medicine. 2nd ed. New York: Oxford University Press; 2017.
Walker S, Griffin S, Asaria M, Tsuchiya A, Sculpher M. Striving for a societal perspective: a framework for economic evaluations when costs and effects fall on multiple sectors and decision makers. Appl Health Econ Health Policy. 2019;17(5):577–90.
Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–78.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7.
World Health Organization. Macroeconomics and health: investing in health for economic development. Geneva: World Health Organization; 2001.
Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(21):2304–22.
The World Bank. GDP per capita (current US$), 2015. 2016. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed 20 Feb 2018.
Phelps CE. A new method to determine the optimal willingness to pay in cost-effectiveness analysis. Value Health. 2019;22(7):785–91.
Snijders TAB, Bosker RJ. Multilevel analysis: an introduction to basic and advanced multilevel modeling. 2nd ed. Los Angeles (CA): Sage Publications; 2012.
Bell CM, Urbach DR, Ray JG, Bayoumi A, Rosen AB, Greenberg D, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332(7543):699–703.
Institute for Clinical and Economic Review. 2020-2023 value assessment framework. Cambdridge: Institute for Clinical and Economic Review; 2020.
Holtgrave DR, Qualls NL, Graham JD. Economic evaluation of HIV prevention programs. Ann Rev Public Health. 1996;17:467–88.
Freedberg KA, Tosteson AN, Cotton JD, Goldman L. Optimal management strategies for HIV-infected patients who present with cough or dyspnea: a cost-effectiveness analysis. J Gen Intern Med. 1992;7:261–72.
Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16:1–31.
Towse A, Pritchard C. National Institute for Clinical Excellence (NICE): is economic appraisal working? Pharmacoeconomics. 2002;20(Suppl. 3):95–105.
Bridges JF, Onukwugha E, Mullins CD. Healthcare rationing by proxy: cost-effectiveness analysis and the misuse of the $50,000 threshold in the US. Pharmacoeconomics. 2010;28(3):175–84.
Braithwaite RS. Willingness to pay for a quality-adjusted life year in search of a standard. Med Care. 2008;46(4):349–56.
Hirth RA. Willingness to pay for a quality-adjusted life year in search of a standard. Med Decis Mak. 2000;20(3):332–42.
Weinstein MC. How much are Americans willing to pay for a quality-adjusted life year? Med Care. 2008;46(4):343–5.
Pauly MV. The questionable economic case for value-based drug pricing in market health systems. Value Health. 2017;20(2):278–82.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this manuscript in terms of conceptualization, data analysis, and finalization of the manuscript in accordance with ICMJE guidelines.
Corresponding author
Ethics declarations
Funding
William Padula is supported by a grant from the National Institutes of Health Office of Extramural Research (KL2 TR001854).
Conflicts of interest/competing interests
William Padula is a consultant for Monument Analytics and is on the scientific advisory board for Molnlycke Healthcare. Charles Phelps is a consultant for Merck, Pfizer, and Audentes Therapeutics. Hui-Han Chen has no conflicts of interest to declare.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Our data are openly available in the published literature through MEDLINE. We have organized these data for public access in the Electronic Supplementary Material provided (Table e1), which aggregate data used for analysis in this study.
Code Availability
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Padula, W.V., Chen, HH. & Phelps, C.E. Is the Choice of Cost-Effectiveness Threshold in Cost-Utility Analysis Endogenous to the Resulting Value of Technology? A Systematic Review. Appl Health Econ Health Policy 19, 155–162 (2021). https://doi.org/10.1007/s40258-020-00606-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-020-00606-4