Abstract
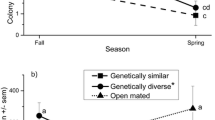
Honey bee queens mate with multiple males resulting in high intracolonial genetic diversity among nestmates; a reproductive strategy known as extreme polyandry. Several studies have demonstrated the adaptive significance of extreme polyandry for overall colony performance and colony growth. Colonies that are more genetically diverse collect more pollen than colonies with less diversity. However, the effects of intracolonial genetic diversity on worker nutritional status are unknown. We created colonies headed by queens instrumentally inseminated with sperm from either 1 or 20 drones, then compared protein consumption, digestion, and uptake among nestmates. We found that nurse bees from colonies with multiple-drone-inseminated (MDI) queens consumed more pollen, had lower amounts of midgut tissue protease, and invested more protein into larvae than nurse bees from single-drone-inseminated (SDI) queens. Pollen foragers from MDI colonies had significantly higher hemolymph protein concentration than pollen foragers from SDI colonies. Differences in hemolymph protein concentration between nurses and pollen foragers were significantly smaller among MDI colonies than among SDI colonies. While intracolonial genetic diversity is correlated with increased foraging, our results suggest that this relationship may be driven in part by the elevated resource demands of nurse bees in genetically diverse colonies that consume and distribute more protein in response to the social context within the hive.





Similar content being viewed by others
References
Alaux, C., Ducloz, F., Crauser, D., Le Conte, Y. (2010) Diet effects on honeybee immunocompetence. Biol. Lett. 6(4), 562–565
Baer, B., Schmid-Hempel, P. (1999) Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397, 151–154
Bitondi, M., Simoes, Z. (1996) The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titers in Africanized Apis mellifera workers. J. Apic. Res. 35(1), 27–36
Blakemore, D., Williams, S., Lehane, M.J. (1995) Protein stimulation of trypsin secretion from the opaque zone midgut cells of Stomoxys calcitrans. Comp. Biochem. Physiol., B: Biochem. Mol Biol 110(2), 301–307
Cappelari, F.A., Turcatto, A.P., Morais, M.M., De Jong, D. (2009) Africanized honey bees more efficiently convert protein diets into hemolymph protein than do Carniolan bees (Apis mellifera carnica). Genet. Mol. Res. 8(4), 1245–1249
Chapman, N., Oldroyd, B., Hughes, W. (2007) Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav. Ecol. Sociobiol. 61(8), 1185–1194
Cole, B.J., Wiernasz, D.C. (1999) The selective advantage of low relatedness. Science 285, 891–893
Crailsheim, K. (1986) Dependence of protein metabolism on age and season in the honeybee (Apis mellifica carnica Pollm). J. Insect Physiol. 32(7), 629–634
Crailsheim, K. (1990) The protein balance of the honey bee worker. Apidologie 21(5), 417–429
Crailsheim, K. (1991) Interadult feeding of jelly in honeybee (Apis mellifera L.) colonies. J. Comp. Physiol B 161(1), 55–60
Crailsheim, K. (1998) Trophallactic interactions in the adult honeybee. Apidologie 29, 97–112
Crailsheim, K., Schneider, L.H.W., Hrassnigg, N., Buhlmann, G., Brosch, U., Gmeinbauer, R., Schoffmann, B. (1992) Pollen consumption and utilization in worker honeybees (Apis mellifera carnica): dependence on individual age and function. J. Insect Physiol. 38(6), 409–419
Cremonez, T., DeJong, D., Bitondi, M. (1998) Quantification of hemolymph proteins as a fast method for testing protein diets for honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91, 1284–1269
da Cruz-Landim, C., Roat, T.C., Fernadez, F.C. (2012) Virus present in the reproductive tract of asymptomatic drones of honey bee (Apis mellifera L.), and possible infection of queen during mating. Microsc. Res. Tech. 75, 986–990
Dadd, R.H. (1956) Proteolytic activity of the midgut in relation to feeding in the beetles Tenebrio molitor L. and Dytiscus marginalis L. J. Exp. Biol 33(2), 311–324
de Miranda, J.R., Fries, I. (2008) Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invert. Pathol. 98, 184–189
DeGrandi-Hoffman, G., Eckholm, B.J., Huang, M.H. (2013) A comparison of bee bread made by Africanized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie 44(1), 52–63
Dreller, C. (1998) Division of labor between scouts and recruits: genetic influence and mechanisms. Behav. Ecol. Sociobiol. 43, 191–196
Eckholm, B.J., Anderson, K.E., Weiss, M., DeGrandi-Hoffman, G. (2011) Intracolonial genetic diversity in honeybee (Apis mellifera) colonies increases pollen foraging efficiency. Behav. Ecol. Sociobiol. 65(5), 1037–1044
Fluri, P., Luscher, M., Wille, H., Gerig, L. (1982) Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 28(1), 61–68
Frumhoff, P.C., Baker, J. (1988) A genetic component to division of labour within honey bee colonies. Nature 333(6171), 358–361
Fuchs, S., Schade, V. (1994) Lower performance in honeybee colonies of uniform paternity. Apidologie 25, 155–168
Giray, T., Guzmán-Novoa, E., Aron, C.W., Zelinsky, B., Fahrbach, S.E., Robinson, G.E. (2000) Genetic variation in worker temporal polyethism and colony defensiveness in the honey bee. Apis mellifera. Behav. Ecol. 11(1), 44–55
Goodisman, M.A.D., Kovacs, J.L., Hoffman, E.A. (2007) The significance of multiple mating in the social wasp Vespula maculifrons. Evolution 61, 2260–2267
Haberl, M., Moritz, R.F.A. (1994) Estimation of intracolonial worker relationship in a honey bee colony (Apis mellifera L.) using DNA fingerprinting. Insectes Soc 41(3), 263–272
Huang, Z.Y., Robinson, G.E. (1992) Honeybee colony integration: worker-worker interactions mediate hormonally regulated plasticity in division of labor. Proc. Natl. Acad. Sci. USA 89(24), 11726–11729
Huang, Z.Y., Robinson, G.E. (1996) Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 39(3), 147–158
Huang, Z.Y., Robinson, G.E. (1999) Social control of division of labor in honey bee colonies. In: Detrain, C., Deneubourg, J.L., Pasteels, J.M. (eds.) Information processing in social insects, pp. 165–186. Birkhauser, Basel, Switzerland
Hughes, W.O.H., Boomsma, J.J. (2004) Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution 58(6), 1251–1260
Hughes, W.O.H., Oldroyd, B.P., Beekman, M., Ratnieks, F.L.W. (2008) Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216
Jones, J.C., Myerscough, M.R., Graham, S., Oldroyd, B.P. (2004) Honey bee nest thermoregulation: diversity promotes stability. Science 305(5682), 402–404
Keller, L., Reeve, H.K. (1994) Genetic variability, queen number, and polyandry in social Hymenoptera. Evolution 48, 694–704
Kraus, B.F., Gerecke, E., Moritz, R.F.A. (2011) Shift work has a genetic basis in honeybee pollen foragers (Apis mellifera L.). Behav. Genet. 41, 323–328
Mattila, H.R., Seeley, T.D. (2007) Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362–364
Mattila, H.R., Seeley, T.D. (2010) Promiscuous honeybee queens generate colonies with a critical minority of waggle-dancing foragers. Behav. Ecol. Sociobiol. 64(5), 875–889
Mattila, H.R., Seeley, T.D. (2011) Does a polyandrous honeybee queen improve through patriline diversity the activity of her colony’s scouting foragers? Behav. Ecol. Sociobiol. 65(4), 799–811
Mattila, H.R., Seeley, T.D. (2014) Extreme polyandry improves a honey bee colony’s ability to track dynamic foraging opportunities via greater activity of inspecting bees. Apidologie 45(3), 347–363
Müller, H.M., Catteruccia, F., Vizioli, J., Della Torre, A., Crisanti, A. (1995) Constitutive and blood meal-induced trypsin genes in Anopheles gambiae. Exp. Parasitol. 81(3), 371–385
Oldroyd, B.P., Clifton, M.J., Parker, K., Wongsiri, S., Rinderer, T.E., Crozier, R.H. (1998) Evolution of mating behavior in the genus Apis and an estimate of mating frequency in Apis cerana (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 91, 700–709
Oldroyd, B.P., Fewell, J.H. (2007) Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22(8), 408–413
Oldroyd, B.P., Rinderer, T.E., Buco, S.M. (1992a) Intra-colonial foraging specialism by honey bees (Apis mellifera) (Hymenoptera: Apidae). Behav. Ecol. Sociobiol. 30(5), 291–295
Oldroyd, B.P., Rinderer, T.E., Buco, S.M., Beaman, L.D. (1993) Genetic variance in honey bees for preferred foraging distance. Anim. Behav. 45(2), 323–332
Oldroyd, B.P., Rinderer, T.E., Harbo, J.R., Buco, S.M. (1992b) Effects of genetic diversity on honey bee (Hymenoptera: Apidae) colony performance. Ann. Entomol. Soc. 85, 335–343
Oster, G.F., Wilson, E.O. (1978) Caste and ecology in the social insects. Monogr. Popul. Biol. 12, 1–352
Palmer, K.A., Oldroyd, B.P. (2003) Evidence for intra-colonial genetic variance in resistance to American foulbrood of honey bees (Apis mellifera): further support for the parasite/pathogen hypothesis for the evolution of polyandry. Naturwissenschaften 90(6), 265–268
Page, R.E., Laidlaw, H.H. (1988) Full sisters and super sisters: a terminological paradigm. Anim. Behav. 36(3), 944–945
Ponton, F., Wilson, K., Cotter, S.C., Raubenheimer, D., Simpson, S.J. (2011) Nutritional immunology: a multi-dimensional approach. PLoS Pathog. 7(12), e1002223
Ribbands, C.R. (1952) Division of labour in the honeybee community. Proc. R. Soc. Lond., B: Biol. Sci 140(898), 32–43
Robinson, G.E. (1992) Regulation of division of labor in insect societies. Annu. Rev. Entomol. 37(1), 637–665
Robinson, G.E., Page, R.E. (1988) Genetic determination of guarding and undertaking in honey-bee colonies. Nature 333, 356–358
Robinson, G.E., Page, R.E. (1989) Genetic basis for division of labor in an insect society. In: Breed, M.D., Page, R.E. (eds.) The genetics of social evolution, pp. 61–80. Westview Press, Boulder, CO
Schlüns, H., Moritz, R.F.A., Neumann, P., Kryger, P., Koeniger, G. (2005) Multiple nuptial flights, sperm transfer and the evolution of extreme polyandry in honeybee queens. Anim. Behav. 70, 125–131
Schmickl, T., Crailsheim, K. (2002) How honeybees (Apis mellifera L.) change their broodcare behaviour in response to non-foraging conditions and poor pollen conditions. Behav. Ecol. Sociobiol 51(5), 415–425
Schmid-Hempel, P. (1998) Parasites in social insects. Princeton Univ. Press, Princeton, NJ
Schmid-Hempel, P. (2005) Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551
Schneider, D. (2009) Physiological integration of innate immunity. In: Rolff, J., Reynolds, S. (eds.) Insect infection and immunity: evolution, ecology, and mechanisms, pp. 106–116. Oxford University Press, Oxford, UK
Schulz, D.J., Huang, Z.Y., Robinson, G.E. (1998) Effects of colony food shortage on behavioral development in honey bees. Behav. Ecol. Sociobiol. 42(5), 295–303
Seeley, T.D. (1985) Honeybee ecology: a study of adapatation in social life. Princeton Univ. Press, Princeton, NJ
Seeley, T.D., Tarpy, D.R. (2007) Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. Lond., B: Biol. Sci 274(1606), 67–72
Tarpy, D.R. (2003) Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. Proc. R. Soc. Lond. B: Biol. Sci 270(1510), 99–103
Tarpy, D.R., Nielsen, R., Nielsen, D.I. (2004) A scientific note on the revised estimates of paternity frequency in Apis. Insectes Soc. 51(2), 203–204
Tarpy, D.R., Page Jr., R.E. (2000) No behavioral control over mating frequency in queen honey bees (Apis mellifera L.). Am. Nat. 155, 820–827
Tarpy, D.R., Seeley, T.D. (2006) Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 93(4), 195–199
Toth, A.L., Kantarovich, S., Meisel, A.F., Robinson, G.E. (2005) Nutritional status influences socially regulated foraging ontogeny in honey bees. J. Exp. Biol. 208, 4641–4649
Toth, A.L., Robinson, G.E. (2005) Worker nutrition and division of labour in honeybees. Anim. Behav. 69(2), 427–435
Wiernasz, D.C., Hines, J., Parker, D.G., Cole, B.J. (2008) Mating for variety increases foraging activity in the harvester ant, Pogonomyrmex occidentalis. Mol. Ecol. 17, 1137–1144
Wiernasz, D.C., Perroni, C.L., Cole, B.J. (2004) Polyandry and fitness in the western harvester ant, Pogonomyrmex occidentalis. Mol. Ecol. 13, 1601–1606
Winston, M.L. (1987) The biology of the honey bee. Harvard University Press, Cambridge, MA
Zakaria, M.E. (2007) Factors affecting on the food metabolism in some honey bee races. J. Appl. Sci. Res. 3(4), 311–316
Zar, J.H. (1999) Biostatistical analysis. Prentice Hall, Inc., Upper Saddle River, NJ
Acknowledgments
We thank Tom Glenn for producing the instrumentally inseminated queens and Dave Mendes for providing colonies. We also thank the USDA-ARS Carl Hayden Bee Research Center technical staff for their help with sample collection and laboratory processing. John Bear, Vanessa Corby-Harris, Linda Eckholm, and three anonymous reviewers provided helpful feedback on the manuscript. This study complied with current guidelines and regulation concerning subject handling. The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: David Tarpy
La diversité génétique à l’intérieur d’une colonie d’abeilles (Apis mellifera) influence le statut nutritionnel des ouvrières
Polyandrie extrême / nutrition / abeille / approvisionnement / protéine
Die genetische Diversität innerhalb von Völkern der Honigbiene (Apis mellifera) beeinflusst den Ernährungszustand der Arbeiterinnen
genetische Diversität innerhalb des Volks / extreme Polyandrie / Ernährung der Honigbiene
Rights and permissions
About this article
Cite this article
Eckholm, B.J., Huang, M.H., Anderson, K.E. et al. Honey bee (Apis mellifera) intracolonial genetic diversity influences worker nutritional status. Apidologie 46, 150–163 (2015). https://doi.org/10.1007/s13592-014-0311-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-014-0311-4