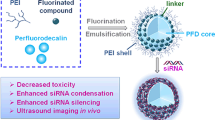
Abstract
Polyethyleneimine (PEI) has been extensively investigated as an efficient carrier for nucleic acid delivery. Yet, it suffers from a high toxicity profile that hinders clinical translation. Fluorination has proven to be a valid approach to reduce the cytotoxicity of PEI and improve the in vitro siRNA delivery potency. Hydrophobicity and lipophobicity can be controllably introduced into the side chains of PEI. However, the effect of fluorination on siRNA delivery in vivo, particularly the biodistribution of siRNA polyplex nanoparticles with fluorinated PEIs, has not been extensively explored. Here, we introduce two series of fluorinated PEIs via amidation with ethyl trifluoroacetate and perfluorobutyryl chloride. Fluorination substantially improved the performance of PEI for siRNA delivery by reducing the cytotoxicity to MDA-MB-231 cells. Importantly, fluorinated PEI enabled the major accumulation of siRNA polyplex nanoparticles in the liver while non-fluorinated PEI delivered siRNA nanoparticles mainly to the lungs after intravenous administration to mice. It is envisioned that fluorination may be an important general strategy for lowering toxicity of cationic polymers, and that the fluorination-induced alteration of biodistribution may be applicable for improved delivery to different organs.

Graphical abstract





Similar content being viewed by others
Data availability
Materials, methods, and additional figures are included in the Supplementary Material and available on the website.
References
Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–38.
Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–93.
Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–77.
Kim HJ, Kim A, Miyata K, Kataoka K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv Drug Deliv Rev. 2016;104:61–77.
Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44(14):6518–48.
Zhou K, Nguyen LH, Miller JB, Yan Y, Kos P, Xiong H, et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc Natl Acad Sci U S A. 2016;113(3):520–5.
Hao J, Kos P, Zhou K, Miller JB, Xue L, Yan Y, et al. Rapid synthesis of a lipocationic polyester library via ring-opening polymerization of functional valerolactones for efficacious siRNA delivery. J Am Chem Soc. 2015;137(29):9206–9.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37.
Yan Y, Xiong H, Zhang X, Cheng Q, Siegwart DJ. Systemic mRNA delivery to the lungs by functional polyester-based carriers. Biomacromolecules. 2017;18(12):4307–15.
Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective ORgan Targeting (SORT) nanoparticles for tissue specific mRNA delivery and CRISPR/Cas gene editing. Nat Nanotechnol. 2020;15(4):313–20.
Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–27.
Philipp A, Zhao XB, Tarcha P, Wagner E, Zintchenko A. Hydrophobically modified oligoethylenimines as highly efficient transfection agents for siRNA delivery. Bioconjug Chem. 2009;20(11):2055–61.
Schroeder A, Dahlman JE, Sahay G, Love KT, Jiang S, Eltoukhy AA, et al. Alkane-modified short polyethyleneimine for siRNA delivery. J Control Release. 2012;160(2):172–6.
Yan Y, Zhou K, Xiong H, Miller JB, Motea EA, Boothman DA, et al. Aerosol delivery of stabilized polyester-siRNA nanoparticles to silence gene expression in orthotopic lung tumors. Biomaterials. 2017;118:84–93.
Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, et al. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc Natl Acad Sci U S A. 2011;108(32):12996–3001.
Kim M-C, Lin MM, Sohn Y, Kim J-J, Kang BS, Kim DK. Polyethyleneimine-associated polycaprolactoneSuperparamagnetic iron oxide nanoparticles as a gene delivery vector. J Biomed Mater Res B. 2017;105(1):145–54.
Bus T, Englert C, Reifarth M, Borchers P, Hartlieb M, Vollrath A, et al. 3rd generation poly(ethylene imine)s for gene delivery. J Mater Chem B. 2017;5(6):1258–74.
Thomas M, Lu JJ, Ge Q, Zhang CC, Chen JZ, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102(16):5679–84.
Schaffert D, Troiber C, Salcher EE, Frohlich T, Martin I, Badgujar N, et al. Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew Chem Int Ed. 2011;50(38):8986–9.
Geihe EI, Cooley CB, Simon JR, Kiesewetter MK, Edward JA, Hickerson RP, et al. Designed guanidinium-rich amphipathic oligocarbonate molecular transporters complex, deliver and release siRNA in cells. Proc Natl Acad Sci U S A. 2012;109(33):13171–6.
Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9(8):648–55.
Brissault B, Leborgne C, Scherman D, Guis C, Kichler A. Synthesis of poly(propylene glycol)-block-polyethylenimine triblock copolymers for the delivery of nucleic acids. Macromol Biosci. 2011;11(5):652–61.
Boisdron-Celle M, Menei PH, Benoit JP. Preparation and characterization of 5-fluorouracil-loaded microparticles as biodegradable anticancer drug carriers. J Pharm Pharmacol. 1995;47(2):108–14.
Jud L, Košutić M, Schwarz V, Hartl M, Kreutz C, Bister K, et al. Expanding the scope of 2′-SCF3 modified RNA. Chem Eur J. 2015;21(29):10400–7.
Wang M, Liu H, Li L, Cheng Y. A fluorinated dendrimer achieves excellent gene transfection efficacy at extremely low nitrogen to phosphorus ratios. Nat Commun. 2014;5(1):3053.
Lv J, Chang H, Wang Y, Wang MM, Xiao JR, Zhang Q, et al. Fluorination on polyethylenimine allows efficient 2D and 3D cell culture gene delivery. J Mater Chem B. 2015;3(4):642–50.
Wu T, Wang L, Ding S, You Y. Fluorinated PEG‐polypeptide polyplex micelles have good serum‐resistance and low cytotoxicity for gene delivery. Macromol Biosci. 2017;17(8):1700114.
Cai XJ, Jin RR, Wang JL, Yue D, Jiang Q, Wu Y, et al. Bioreducible fluorinated peptide dendrimers capable of circumventing various physiological barriers for highly efficient and safe gene delivery. ACS Appl Mater Interfaces. 2016;8(9):5821–32.
Fisicaro E, Compari C, Bacciottini F, Contardi L, Pongiluppi E, Barbero N, et al. Nonviral gene-delivery by highly fluorinated gemini bispyridinium surfactant-based DNA nanoparticles. J Colloid Interface Sci. 2017;487:182–91.
Wang LH, Wu DC, Xu HX, You YZ. High DNA-binding affinity and gene-transfection efficacy of bioreducible cationic nanomicelles with a fluorinated core. Angew Chem Int Ed. 2016;55(2):755–9.
Chen G, Wang K, Ullah A, Zhou Y, Ding L, Hu Q, et al. Self-assembled fluoropolycation nanomicelles for improved siRNA silencing. J Control Release. 2017;259:e35–e6.
Liu X, Wang Y, Chen C, Tintaru A, Cao Y, Liu J, et al. A fluorinated bola-amphiphilic dendrimer for on-demand delivery of siRNA, via specific response to reactive oxygen species. Adv Funct Mater. 2016;26(47).
Chen G, Wang K, Hu Q, Ding L, Yu F, Zhou Z, et al. Combining fluorination and bioreducibility for improved siRNA polyplex delivery. ACS Appl Mater Interfaces. 2017;9(5):4457–66.
Cai X, Zhu H, Zhang Y, Gu Z. Highly efficient and safe delivery of VEGF siRNA by bioreducible fluorinated peptide dendrimers for cancer therapy. ACS Appl Mater Interfaces. 2017;9(11):9402–15.
Wang H, Wang Y, Wang Y, Hu J, Li T, Liu H, et al. Self-assembled fluorodendrimers combine the features of lipid and polymeric vectors in gene delivery. Angew Chem Int Ed. 2015;54(40):11647–51.
Gong J-H, Wang Y, Xing L, Cui P-F, Qiao J-B, He Y-J, et al. Biocompatible fluorinated poly(beta-amino ester)s for safe and efficient gene therapy. Int J Pharm. 2018;535(1-2):180–93.
Xiao Y-P, Zhang J, Liu Y-H, Huang Z, Wang B, Zhang Y-M, et al. Cross-linked polymers with fluorinated bridges for efficient gene delivery. J Mater Chem B. 2017;5(43):8542–53.
Johnson ME, Shon J, Guan BM, Patterson JP, Oldenhuis NJ, Eldredge AC, et al. Fluorocarbon modified low-molecular-weight polyethylenimine for siRNA delivery. Bioconjug Chem. 2016;27(8):1784–8.
Wang MM, Cheng YY. Structure-activity relationships of fluorinated dendrimers in DNA and siRNA delivery. Acta Biomater. 2016;46:204–10.
Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51.
Yan Y, Liu L, Xiong H, Miller JB, Zhou K, Kos P, et al. Functional polyesters enable selective siRNA delivery to lung cancer over matched normal cells. Proc Natl Acad Sci U S A. 2016;113(39):E5702–E10.
Gunther M, Lipka J, Malek A, Gutsch D, Kreyling W, Aigner A. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2011;77(3):438–49.
Gao S, Dagnaes-Hansen F, Nielsen EJB, Wengel J, Besenbacher F, Howard KA, et al. The effect of chemical modification and nanoparticle formulation on stability and biodistribution of siRNA in mice. Mol Ther. 2009;17(7):1225–33.
Funding
D.J.S. received financial support from the Cancer Prevention and Research Institute of Texas (CPRIT) (R1212), the Welch Foundation (I-1855), the American Cancer Society (RSG-17-012-01), and the Mary Kay Foundation (049-15). Y.Y. received financial support from Zhejiang Provincial Natural Science Foundation of China (LY19B040004).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 144 kb)
Rights and permissions
About this article
Cite this article
Xue, L., Yan, Y., Kos, P. et al. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv. and Transl. Res. 11, 255–260 (2021). https://doi.org/10.1007/s13346-020-00790-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00790-9