Abstract
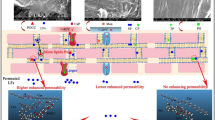
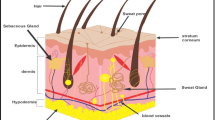
Transdermal drug delivery is advantageous over other conventional drug administration routes. However, it can be inefficient because of the natural barrier of the stratum corneum which is the uppermost layer of the skin. A previous study verified that the treatment of magainin pore-forming peptide with N-lauroylsarcosine (NLS) on human skin can increase skin permeability by 47-fold. However, NLS is well known as a potential skin irritant. The irritation potential of NLS is known to decrease when mixed with sorbitan monolaurate (S20). Encouraged by these results, we combined S20 with magainin-NLS to enhance transdermal drug transport with less skin irritation. In this study, nine groups with magainin and NLS:S20 mixtures at different concentrations and weight fractions were screened to maximize their synergistic effect. To quantify the efficacy to toxicity ratio of each formulation, we defined the ratio as the “enhancement ratio/irritation potential (ER/IP).” The ER was observed by Franz cell diffusion of the target drug fluorescein, and the IP was measured by the cytotoxicity of the NIH/3T3 mouse fibroblast cell line. As a result, the magainin with the NLS:S20 mixture increased the permeability of porcine skin as well as decreased the toxicity. Among the various combinations, a formulation of 2% (w/v) NLS:S20 with a weight fraction of 0.6:0.4 had the largest ER/IP. ATR-FTIR spectroscopy of the formulations and skin was done to analyze the interactions in the formulations themselves and between the formulations and the skin. Both the intercellular lipidic route and transcellular route through the stratum corneum protein were involved in the delivery of fluorescein. This study turned pore-forming peptides into an efficient and safe penetration enhancer by combining them with other chemical penetration enhancers. Moreover, this discovery could be a possible method for enabling the transdermal delivery of macromolecules.




Similar content being viewed by others
References
Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3:115–24.
Kim Y-C, Late S, Banga AK, Ludovice PJ, Prausnitz MR. Biochemical enhancement of transdermal delivery with magainin peptide: modification of electrostatic interactions by changing pH. Int J Pharm. 2008;362:20–8.
Prausnitz MR, Elias PM, Franz TJ, Schmuth M, Tsai J-C, Menon GK, et al. Skin barrier and transdermal drug delivery. Dermatology. 2012;3:2065–73.
Wertz PW. Lipids and barrier function of the skin. Acta Derm Venereol Suppl. 2000;208:7–11.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37.
Nasrollahi SA, Taghibiglou C, Azizi E, Farboud ES. Cell-penetrating peptides as a novel transdermal drug delivery system. Chem Biol Drug Design. 2012;80:639–46.
Singh G, Karande P. Peptide-mediated transdermal drug delivery. In: Dragicevic N, Maibach HI, editors. Percutaneous penetration enhancers chemical methods in penetration enhancement. Berlin Heidelberg: Springer; 2015. p. 353–61.
Kumar S, Narishetty ST, Tummala H. Peptides as skin penetration enhancers for low molecular. In: Dragicevic N, Maibach HI, editors. Percutaneous penetration enhancers chemical methods in penetration enhancement. Berlin Heidelberg: Springer; 2015. p. 337–52.
Kumar S, Zakrewsky M, Chen M, Menegatti S, Muraski JA, Mitragotri S. Peptides as skin penetration enhancers: mechanisms of action. J Control Release. 2015;199:168–78. https://doi.org/10.1016/j.jconrel.2014.12.006.
Menegatti S, Zakrewsky M, Kumar S, De Oliveira JS, Muraski JA, Mitragotri S. De novo design of skin-penetrating peptides for enhanced transdermal delivery of peptide drugs. Adv Healthc Mater. 2016;5:602–9.
Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by magainin pore-forming peptide. J Control Release. 2007;122:375–83.
Kim YC, Ludovice PJ, Prausnitz MR. Transdermal delivery enhanced by antimicrobial peptides. J Biomed Nanotechnol. 2010;6:612–20.
Kaushik S, Krishnan A, Prausnitz MR, Ludovice PJ. Magainin-mediated disruption of stratum corneum lipid vesicles. Pharm Res. 2001;18:894–6.
Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemist. 1996;35:13723–8.
Hall K, Lee T-H, Mechler AI, Swann MJ, Aguilar M-I. Real-time measurement of membrane conformational states induced by antimicrobial peptides: balance between recovery and lysis. Scient Rep. 2014;4
Sato H, Feix JB. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1245–56. https://doi.org/10.1016/j.bbamem.2006.02.021.
Aioi A, Kuriyama K, Shimizu T, Yoshioka M, Uenoyama S. Effects of vitamin E and squalene on skin irritation of a transdermal absorption enhancer, lauroylsarcosine. Int J Pharm. 1993;93:1–6.
Aioi A, Shimizu T, Kuriyama K. Effect of squalene on superoxide anion generation induced by a skin irritant, lauroylsarcosine. Int J Pharm. 1995;113:159–64.
Shimizu T, Aioi A, Horiguchi T, Kuriyama K. Effect of vitamin E on keratinocyte-modulation induced by lauroylsarcosine. The Japanese Aust J Pharm. 1995;67:291–5.
Karande P, Jain A, Arora A, Ho MJ, Mitragotri S. Synergistic effects of chemical enhancers on skin permeability: a case study of sodium lauroylsarcosinate and sorbitan monolaurate. European J Pharm Sci. 2007;31:1–7.
Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–5.
Jung EC, Maibach HI. Animal models for percutaneous absorption. J Appl Toxicol. 2015;35:1–10.
Welss T, Basketter DA, Schröder KR. In vitro skin irritation: facts and future. State of the art review of mechanisms and models. Toxicol in Vitro. 2004;18:231–43.
Qin G, Geng S, Wang L, Dai Y, Yang B, Wang J-Y. Charge influence of liposome on transdermal delivery efficacy. Soft Matter. 2013;9:5649–56.
Tomankova K, Kejlova K, Binder S, Daskova A, Zapletalova J, Bendova H, et al. In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol in Vitro. 2011;25:1242–50.
Asbill CS, Michniak BB. Percutaneous penetration enhancers: local versus transdermal activity. Pharm Sci Technol Today. 2000;3:36–41.
Gennari CG, Franzè S, Pellegrino S, Corsini E, Vistoli G, Montanari L, et al. Skin penetrating peptide as a tool to enhance the permeation of heparin through human epidermis. Biomacromolecules. 2015;17:46–55.
Cilurzo F, Vistoli G, Selmin F, Gennari CG, Musazzi UM, Franzé S, et al. An insight into the skin penetration enhancement mechanism of N-methylpyrrolidone. Mol Pharm. 2014;11:1014–21.
Kim YC, Ludovice PJ, Prausnitz MR. Optimization of transdermal delivery using magainin pore-forming peptide. J PhyChem Solids. 2008;69:1560–3.
Yuan H, Zhao S, Cheng G, Zhang L, Miao X, Mao S, et al. Mixed micelles of Triton X-100 and cetyl trimethylammonium bromide in aqueous solution studied by 1H NMR. The J Phy Chem B. 2001;105:4611–5.
Lémery E, Briançon S, Chevalier Y, Bordes C, Oddos T, Gohier A, et al. Skin toxicity of surfactants: structure/toxicity relationships. Coll Surf A: Physicochemical Eng ASp. 2015;469:166–79.
Hall-Manning T, Holland G, Rennie G, Revell P, Hines J, Barratt M, et al. Skin irritation potential of mixed surfactant systems. Food and Chemical Toxicology. 1998;36:233–8.
Huang HW, Chen F-Y, Lee M-T. Molecular mechanism of peptide-induced pores in membranes. Phy Rev Lett. 2004;92:198304.
Matsuzaki K, Sugishita K-i, Harada M, Fujii N, Miyajima K. Interactions of an antimicrobial peptide, magainin 2, with outer and inner membranes of Gram-negative bacteria. Biochim Biophy Acta (BBA)-Biomembranes. 1997;1327:119–30.
Thind R, O'Neill D, Del Regno A, Notman R. Ethanol induces the formation of water-permeable defects in model bilayers of skin lipids. Chem Comm. 2015;51:5406–9.
Acknowledgements
Porcine skin was kindly donated by the Heart Research Center of Chonnam National University.
Funding
This work was supported financially by the Ministry of Science, ICT, and Future Planning (Project No. NRF-2014M3A9E4064580); Advanced Biomass R&D Center (ABC) of the Global Frontier Project funded by the Ministry of Science, ICT, and Future Planning (NRF-2015M3A6A2074238); and the KUSTAR-KAIST Institute at KAIST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lee, H., Park, J. & Kim, YC. Enhanced transdermal delivery with less irritation by magainin pore-forming peptide with a N-lauroylsarcosine and sorbitan monolaurate mixture. Drug Deliv. and Transl. Res. 8, 54–63 (2018). https://doi.org/10.1007/s13346-017-0433-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0433-0