Abstract
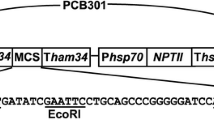
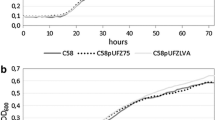
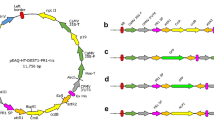
One of many uses of fluorescent proteins in plant pathology is their constitutive expression in a pathogen in order to facilitate microscopic visualization of host-pathogen interactions. However, if such transformants are to be useful, it is important that they be similar to wild type isolates in their ability to cause disease. The vegetable pathogen Phytophthora capsici was transformed to stably and constitutively express genes for either a green (pgfp) or red (tdTomato) fluorescent protein. All transformants fluoresced in all life stages, but varied in their intensity and contained one, two, or five copies of pgfp or tdTomato, as determined by Southern analysis. One transformant labeled with green fluorescent protein had reduced growth on artificial medium, produced smaller lesions on detached pepper fruit, and was reduced in virulence on pepper seedlings, compared to the wild type isolate. For these reasons, it is unsuitable for use in studies of host-pathogen interactions. Based on their intense fluorescence and similarity to the wild type isolate in growth and virulence, the other four transformants will be useful in future microscopy studies of interactions between P. capsici and its various hosts.


Similar content being viewed by others
References
Abu-El Samen FM, Secor GA, Gudmestad NC (2003a) Genetic variation among asexual progeny of Phytophthora infestans detected with RAPD and AFLP markers. Plant Pathol 52:314–325
Abu-El Samen FM, Secor GA, Gudmestad NC (2003b) Variability in virulence among asexual progenies of Phytophthora infestans. Phytopathology 93:293–304
Ah-Fong AMV, Judelson HS (2011) Vectors for fluorescent protein tagging in Phytophthora: tools for functional genomics and cell biology. Fungal Biol 115:882–890
Bailey AM, Mena GL, Herrera-Estrella L (1991) Genetic transformation of the plant pathogens Phytophthora capsici and Phytophthora parasitica. Nucleic Acids Res 19:4273–4278
Bernhardt EA, Grogan RG (1982) Effect of soil matric potential on the formation and indirect germination of sporangia of Phytophthora parasitica, Phytophthora capsici, and Phytophthora cryptogea. Phytopathology 72:507–511
Café-Filho AC, Duniway JM (1995) Dispersal of Phytophthora capsici and P. parasitica in furrow-irrigated rows of bell pepper, tomato and squash. Plant Pathol 44:1025–1032
Chen N, Hsiang T, Goodwin PH (2003) Use of green fluorescent protein to quantify the growth of Colletotrichum during infection of tobacco. J Microbiol Methods 53:113–122
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Crossan DF, Haasis FA, Ellis DE (1954) Phytophthora blight of summer squash. Plant Dis Report 38:557–559
Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY (1995) Understanding, improving and using green fluorescent proteins. Trends Biochem Sci 20:448–455
Czymmek KJ, Bourett TM, Howard RJ (2004) Fluorescent protein probes in fungi. Methods Microbiol 34:27–62
Davidson CR, Carroll RB, Evan TA, Mulrooney RP (2002) First report of Phytophthora capsici infecting lima bean (Phaseolus lunatis) in the mid-Atlantic region. Plant Dis 86:1049
Dumas B, Centis S, Sarrazin N, Esquerre-Tugaye MT (1999) Use of green fluorescent protein to detect expression of an endopolygalacturonase gene of Colletotrichum lindemuthianum during bean infection. Appl Environ Microbiol 65:1769–1771
Dunn AR, Milgroom MG, Meitz JC, McLeod A, Fry WE, McGrath MT, Dillard HR, Smart CD (2010) Population structure and resistance to mefenoxam of Phytophthora capsici in New York State. Plant Dis 94:1461–1468
Erwin DC, Ribeiro OK (1996) Phytophthora diseases worldwide. The American Phytopathological Society, St. Paul
Foster JM, Hausbeck MK (2010) Resistance of pepper to Phytophthora crown, root, and fruit rot is affected by isolate virulence. Plant Dis 94:24–30
French-Monar RD, Jones JB, Roberts PD (2006) Characterization of Phytophthora capsici associated with roots of weeds on Florida vegetable farms. Plant Dis 90:345–350
Gevens AJ, Ando K, Lamour KH, Grumet R, Hausbeck MK (2006) A detached cucumber fruit method to screen for resistance to Phytophthora capsici and effect of fruit age on susceptibility to infection. Plant Dis 90:1276–1282
Gevens AJ, Donahoo RS, Lamour KH, Hausbeck MK (2008) Characterization of Phytophthora capsici causing foliar and pod blight of snap beans in Michigan. Plant Dis 92:201–209
Granke LL, Windstam ST, Hoch HC, Smart CD, Hausbeck MK (2009) Dispersal and movement mechanisms of Phytophthora capsici sporangia. Phytopathology 99:1258–1264
Hausbeck MK, Lamour KH (2004) Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Dis 88:1292–1303
Heim R, Cubitt AB, Tsien RY (1995) Improved green fluorescence. Nature 373:663–664
Hoch H, Galvani C, Szarowski D, Turner J (2005) Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia 97:580–588
Judelson H, Tyler B, Michelmore R (1991) Transformation of the oomycete pathogen, Phytophthora infestans. Mol Plant Microbe Interact 4:602–607
Judelson HS, Dudler R, Pieterse CMJ, Unkles SE, Michelmore RW (1993) Expression and antisense inhibition of transgenes in Phytophthora infestans is modulated by choice of promoter and position effects. Gene 133:63–69
Keller NP, Bergstrom GC, Yoder OC (1990) Effects of genetic transformation on fitness of Cochliobolus heterostrophus. Phytopathology 80:1166–1173
Lagopodi A, Ram A, Lamers G, Punt P, Van den Hondel C, Lugtenberg B, Bloemberg G (2002) Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol Plant Microbe Interact 15:172–179
Lamour KH, Hausbeck MK (2003) Effect of crop rotation on the survival of Phytophthora capsici in Michigan. Plant Dis 87:841–845
Larrainzar E, O’Gara F, Morrissey JP (2005) Applications of autofluorecent proteins for in situ studies in microbial ecology. Annu Rev Microbiol 59:257–277
Li C, Chen S, Zuo C, Sun Q, Ye Q, Yi G, Huang B (2011) The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp cubense race 4. Eur J Plant Pathol 131:327–340
Lorang JM, Tuori RP, Martinez JP, Sawyer TL, Redman RS, Rollans JA, Wolpert TJ, Johnson KB, Rodriguez RJ, Dickman MB, Ciuffetti LM (2001) Green fluorescent protein is lighting up fungal biology. Appl Environ Microbiol 67:1987–1994
Madden LV, Hughes G, van den Bosch F (2007) The study of plant disease epidemics. The American Phytopathological Society, St. Paul
Maor R, Puyesky M, Horwitz B, Sharon A (1998) Use of green fluorescent protein (GFP) for studying development and fungal-plant interaction in Cochliobolus heterostrophus. Mycol Res 102:491–496
Mauch F, Torche S, Schläppi K, Branciard L, Belhaj K, Parisy V, Si-Ammour A (2009) Phytophthora brassicae as a pathogen of arabidopsis. In: Lamour KH, Kamoun S (eds) Oomycete genetics and genomics: diversity, interactions, and research tools. Wiley-Blackwell, Hoboken, p 574
McLeod A, Fry BA, Zuluaga AP, Myers KL, Fry WE (2008) Toward improvements of oomycete transformation protocols. J Eukaryot Microbiol 55:103–109
Nahalkova J (2003) Red fluorescent protein (DsRed2) as a novel reporter in Fusarium oxysporum f. sp. lycopersici. FEMS Microbiol Lett 225:305–309
Njoroge SMC, Vallad GE, Park S-Y, Kang S, Koike ST, Bolda M, Burman P, Polonik W, Subbarao KV (2011) Phenological and phytochemical changes correlate with differential interactions of Verticillium dahliae with broccoli and cauliflower. Phytopathology 101:523–534
Pang SZ, DeBoer DL, Wan Y, Ye G, Layton JG, Neher MK, Armstrong CL, Fry JE, Hinchee MA, Fromm ME (1996) An improved green fluorescent protein gene as a vital marker in plants. Plant Physiol 112:893–900
Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ (1992) Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111:229–233
Quesada-Ocampo LM, Fulbright DW, Hausbeck MK (2009) Susceptibility of Fraser Fir to Phytophthora capsici. Plant Dis 93:135–141
Riedel M, Calmin G, Belbahri L, Lefort F, Götz M, Wagner S, Werres S (2009) Green fluorescent protein (GFP) as a reporter gene for the plant pathogenic oomycete Phytophthora ramorum. J Eukaryot Microbiol 56:130–135
Ristaino JB (1991) Influence of rainfall, drip irrigation, and inoculum density on the development of Phytophthora root and crown rot epidemics and yield in bell pepper. Phytopathology 81:922–929
Ristaino JB, Johnston SA (1999) Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Dis 83:1080–1089
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, vol 1, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schmitthenner AF, Bhat RG (1994) Useful methods for studying Phytophthora in the laboratory. Department of Plant Pathology, Ohio Agricultural Research and Development Center, The Ohio State University, Wooster
Schornack S, van Damme M, Bozkurt TO, Cano L, Smoker M, Thines M, Gaulin E, Kamoun S, Huitema E (2010) Ancient class of translocated oomycete effectors targets the host nucleus. Proc Natl Acad Sci 107:17421–17426
Sexton A, Howlett J (2001) Green fluorescent protein as a reporter in the Brassica-Leptosphaeria maculans interaction. Physiol Mol Plant Pathol 58:13–21
Shaner NC, Campbell RE, Steinbach PA, Ben NG, Giepmans, Palmer AE, Tsien RY (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22:1567–1572
Si-Ammour A, Mauch-Mani B, Mauch F (2003) Quantification of induced resistance against Phytophthora species expressing GFP as a vital marker: beta-aminobutyric acid but not BTH protects potato and Arabidopsis from infection. Mol Plant Pathol 4:237–248
Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33:775–792
Vallad GE, Subbarao KV (2008) Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 98:871–885
van West P, Reid B, Campbell TA, Sandrock RW, Fry WE, Kamoun S, Gow NAR (1999) Green fluorescent protein (GFP) as a reporter gene for the plant pathogenic oomycete Phytophthora palmivora. FEMS Microbiol Lett 178:71–80
Visser M, Gordon T, Wingfield B, Wingfield M, Viljoen A (2004) Transformation of Fusarium oxysporum f. sp cubense, causal agent of Fusarium wilt of banana, with the green fluorescent protein (GFP) gene. Australas Plant Pathol 33:69–75
Yang HA, Zhou J, Sivasithamparam K, O’Brien PA (1994) Variations in culture morphology and pathogenicity among protoplast-regenerated strains of Rhizoctonia solani. FEMS Microbiol Lett 115:83–86
Acknowledgments
This project was supported by the Agriculture and Food Research Initiative Competitive Grant No. 2012-67011-19690 from the USDA National Institute of Food and Agriculture, and by the New York State Department of Agriculture and Markets through a NY Specialty Crops Block Grant. Support for A. Dunn was also provided by a fellowship from Cornell University College of Agriculture and Life Sciences. The authors thank H. C. Hoch for assistance with the confocal laser scanning microscope; H. Nihart for technical assistance; and the Provost of Hobart and William Smith Colleges for support of H. Nihart.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dunn, A.R., Fry, B.A., Lee, T.Y. et al. Transformation of Phytophthora capsici with genes for green and red fluorescent protein for use in visualizing plant-pathogen interactions. Australasian Plant Pathol. 42, 583–593 (2013). https://doi.org/10.1007/s13313-013-0222-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-013-0222-2