Abstract
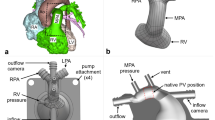
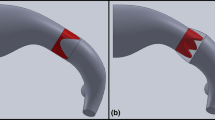
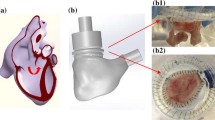
Tetralogy of Fallot is a congenital heart disease characterized over time, after the initial repair, by the absence of a functioning pulmonary valve, which causes regurgitation, and by progressive enlargement of the right ventricle outflow tract (RVOT). Due to this pathological anatomy, available transcatheter valves are usually too small to be deployed there. To avoid surgical valve replacement, an alternative consists in implanting a reducer prior to or in combination with the valve. It has been shown in animal experiments to be promising, but with some limitations. The effect of a percutaneous pulmonary valve reducer on hemodynamics in enlarged RVOT is thus studied by computational modeling. To this aim, blood flow in the RVOT is modeled with CFD coupled to a simplified valve model and 0D downstream models. Simulations are performed in an image-based geometry and boundary conditions tuned to reproduce the pathological flow without the device. Different device designs are built and compared with the initial device-free state, or with the reducer alone. Results suggest that pressure loss is higher for the reducer alone than for the full device, and that the latter successfully restores hemodynamics to a healthy state and induces a more symmetric flow in the pulmonary arteries. Moreover, pressure forces on the reducer and on the valve have the same magnitudes. Migration would occur towards the right ventricle rather than the pulmonary arteries. Results support the thesis that the reducer does not introduce clinically significant pressure gradients, as was found in animal experiments. Such study could help transfer to patients.












Similar content being viewed by others
References
Amahzoune, B., C. Szymansky, J. N. Fabiani, and R. Zegdi. A new endovascular size reducer for large pulmonary outflow tract. Eur. J. Cardio-Thoracic Surg. 37(3):730–732, 2010.
Arbia, G., C. Corsini, C. Baker, G. Pennati, T. Y. Hsia, and I. E. Vignon-Clementel for MOCHA. Pulmonary hemodynamics simulations before stage 2 single ventricle surgery: patient-specific parameter identification and clinical data assessment. Cardiovasc. Eng. Technol., 2015. DOI:10.1007/s13239-015-0212-3.
Astorino, M., J. F. Gerbeau, O. Pantz, and K. F. Traoré. Fluid-structure interaction and multi-body contact. application to the aortic valves. Comput. Methods Appl. Mech. Eng. 198(46–46):3603–3612, 2009.
Astorino, M., J. Hamers, S. Shadden, and J. F. Gerbeau. A robust and efficient valve model based on resistive immersed surfaces. Int. J. Numer. Method Biomed. Eng. 28(9):937–959, 2012.
Bertoglio, C. and A. Caiazzo. A tangential regularization method for backflow stabilization with application to blood flow simulations. J. Comput. Phys. 261(1800):162–171, 2013.
Bonhoeffer, P., Y. Boudjemline, Z. Saliba, A. Hausse, Y. Aggoun, D. Bonnet, D. Sidi, and J. Kachaner. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation 102(7):813–816, 2000.
Botney, M. D. Role of hemodynamics in pulmonary vascular remodeling: implications for primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 159(2):361–4, 1999. DOI:10.1164/ajrccm.159.2.9805075.
Boudjemline, Y., G. Agnoletti, D. Bonnet, D. Sidi, and P. Bonhoeffer. Percutaneous pulmonary valve replacement in a large right ventricular outflow tract: an experimental study. J. Am. Coll. Cardiol. 43(6):1082–1087, 2004. DOI:10.1016/j.jacc.2003.10.037.
Cabrera, M., C. Oomens, C. Bouten, A. Bogers, S. Hoerstrup, and F. Baaijens. Mechanical analysis of ovine and pediatric pulmonary artery for heart valve stent design. J. Biomech. 46(12), 2075–2081, 2013.
Caiazzo, A., M. Fernández, J. F. Gerbeau, and V. Martin. Projection schemes for fluid flows through a porous interface. SIAM J. Sci. Comput. 33(2):541–564, 2011.
Chorin, A. Numerical solution of the Navier-Stokes equations. Math. Comput. 22:745–762, 1968.
Das, A., W. M. Gottliebson, M. Karve, and R. Banerjee. Comparison of hemodynamic endpoints between normal subject and tetralogy patient using Womersley velocity profile and MR based flow measurements. Mol. Cell. Biomech. 8(1):21–42, 2011
Fernández, M., J. F. Gerbeau, and V. Martin. Numerical simulation of blood flows through a porous interface. Math. Mod. Num. An. (M2AN) 42(6):961–990, 2008.
Fogel, M. A., K. S. Sundareswaran, D. de Zelicourt, L. P. Dasi, T. Pawlowski, J. Rome, and A. P. Yoganathan. Power loss and right ventricular efficiency in patients after tetralogy of fallot repair with pulmonary insufficiency: Clinical implications. J. Thoracic Cardiovasc. Surg. 143(6):1279–1285, 2012. DOI:10.1016/j.jtcvs.2011.10.066.
Frank, O. Die Grundform Des Arteriellen Pulses. Zeitung für Biologie 37:483–586, 1899.
Geiger, J., M. Markl, B. Jung, J. Grohmann, B. Stiller, M. Langer, and R. Arnold. 4d-mr flow analysis in patients after repair for tetralogy of fallot. Eur. Radiol. 21:1651–1657, 2011.
George, P., H. Borouchaki, and E. Saltel. Ultimate robustness in meshing an arbitrary polyhedron. Int. J. Numer. Methods Eng. IJNME 58:1061–1089, 2003.
Guermond, J. L., P. Minev, and J. Shen. An overview of projection methods for incompressible flows. Comput. Methods Appl. Mech. Eng. 195:6011–6045, 2006.
Guibert, R., K. Mcleod, A. Caiazzo, T. Mansi, M. A. Fernández, M. Sermesant, X. Pennec, I. E. Vignon-Clementel, Y. Boudjemline, and J. F. Gerbeau. Group-wise construction of reduced models for understanding and characterization of pulmonary blood flows from medical images. Med. Image Anal. 18(1):63–82, 2014.
Kilner, P. J., R. Balossino, G. Dubini, S. V. Babu-Narayan, A. M. Taylor, G. Pennati, and F. Migliavacca. Pulmonary regurgitation: the effects of varying pulmonary artery compliance, and of increased resistance proximal or distal to the compliance. Int. J. Cardiol. 133(2):157–166, 2009. DOI:10.1016/j.ijcard.2008.06.078.
Lurz, P., P. Bonhoeffer, and A. M. Taylor. Percutaneous pulmonary valve implantation: an update. Expert Rev. Cardiovasc. Ther. 7(7):823–33, 2009. DOI:10.1586/erc.09.57.
McLeod, K., A. Caiazzo, M. A. Fernández, T. Mansi, I. E. Vignon-Clementel, M. Sermesant, X. Pennec, Y. Boudjemline, and J. F. Gerbeau. Atlas-based reduced models of blood flows for fast patient-specific simulations. In: Proceedings of MICCAI Workshop on Statistical Atlases and Computational Models of the Heart: Mapping Structure and Function + a Cardiac Electrophysiological Simulation Challenge (STACOM+CESC’10). Lecture Notes in Computer Science, vol. 6364. Beijing: Springer, pp. 95–104, 2010.
Mollet, A., A. Basquin, B. Stos, and Y. Boudjemline. Off-pump replacement of the pulmonary valve in large right ventricular outflow tracts: a transcatheter approach using an intravascular infundibulum reducer. Pediatr. Res. 62(4):428–433, 2007.
Momenah, T. S., R. El Oakley, K. Al Najashi, S. Khoshhal, H. Al Qethamy, and P. Bonhoeffer. Extended application of percutaneous pulmonary valve implantation. J. Am. Coll. Cardiol. 53(20):1859–1863, 2009. DOI:10.1016/j.jacc.2008.08.061.
Pant, S., B. Fabrèges, J. F. Gerbeau, and I. Vignon-Clementel. A methodological paradigm for patient-specific multi-scale cfd simulations: from clinical measurements to parameter estimates for individual analysis. Int. J. Numer. Methods Biomed. Eng. 30(12):1614–1648, 2014.
Prasad, A., L. K. To, M. L. Gorrepati, C. K Zarins, and C. A. Figueroa. Computational analysis of stresses acting on intermodular junctions in thoracic aortic endografts. J. Endovasc. Ther. 18(4):559–68, 2011. DOI:10.1583/11-3472.1.
Preston-Maher, G. L., R. Torii, and G. Burriesci. A technical review of minimally invasive mitral valve replacements. Cardiovasc. Eng. Technol., 2014.
Schievano, S., L. Coats, F. Migliavacca, W. Norman, A. Frigiola, J. Deanfield, P. Bonhoeffer, and A. M. Taylor. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J. Cardiovasc. Magn. Reson. 9(4):687–695, 2007. DOI:10.1080/10976640601187596.
Schievano, S., A. M. Taylor, C. Capelli, P. Lurz, J. Nordmeyer, F. Migliavacca, and P. Bonhoeffer. Patient specific finite element analysis results in more accurate prediction of stent fractures: application to percutaneous pulmonary valve implantation. J. Biomech. 43(4):687–693, 2010. DOI:10.1016/j.jbiomech.2009.10.024.
Si, H. TetGen: A Quality Tetrahedral Mesh Generator and a 3D Delaunay Triangulator. Tech. Rep. 1762, WIAS, Berlin, 2013.
Tang, D., C. Yang, T. Geva, and P. J. Del Nido. Patient-specific mri-based 3d fsi rv/lv/patch models for pulmonary valve replacement surgery and patch optimization. J. Biomech. Eng. 130(4):041,010, 2008. 18601452.
Tang, B. T., T. A. Fonte, F. P. Chan, P. S. Tsao, J. A. Feinstein, and C. A. Taylor. Three-dimensional hemodynamics in the human pulmonary arteries under resting and exercise conditions. Ann. Biomed. 39(1):347–58, 2011
Temam, R. Une méthode d’approximation de la solution des équations de Navier-Stokes. Bull. Soc. Math. France 96:115–152, 1968.
Troianowski, G., C. A. Taylor, J. A. Feinstein, and I. E. Vignon-Clementel. Three-dimensional simulations in glenn patients: clinically based boundary conditions, hemodynamic results and sensitivity to input data. J. Biomech. Eng. Trans. Asme 133(11), 2011.
Vignon-Clementel, I., C. Figueroa, K. Jansen, and C. Taylor. Outflow boundary conditions for 3D simulations of non-periodic blood flow and pressure fields in deformable arteries. Comput. Method Biomech. Biomed. Eng. 111(3):502–513, 2010.
Vignon-Clementel, I.E., A. L. Marsden, and J. A. Feinstein. A primer on computational simulation in congenital heart disease for the clinician. Prog. Pediatr. Cardiol. 30(1–2):3–13, 2010. DOI:10.1016/j.ppedcard.2010.09.002.
Voser, E. M., C. J. Kellenberger, and E. R. V. Buechel. Effects of pulmonary regurgitation on distensibility and flow of the branch pulmonary arteries in tetralogy of fallot. Pediatr. Cardiol. 34:1118–1124, 2013.
Yang, W., I. E. Vignon-Clementel, G. Troianowski, V. M. Reddy, J. A. Feinstein, and A. L Marsden. Hepatic blood flow distribution and performance in conventional and novel Y-graft Fontan geometries: a case series computational fluid dynamics study. J. Thorac. Cardiovasc. Surg., 2011. DOI:10.1016/j.jtcvs.2011.06.042.
Yun, B. M., L. P. Dasi, C. K. Aidun, and A. P. Yoganathan. Computational modelling of flow through prosthetic heart valves using the entropic lattice-boltzmann method. J. Fluid Mech. 743:170–201, 2014. DOI:10.1017/jfm.2014.54.
Acknowledgments
The authors gratefully acknowledge Kristin McLeod, formerly at Asclepios project team, INRIA Sophia-Antipolis Mediterranée, France, now at Simula Research Laboratory, Oslo, for the device-free geometry reconstruction from MRI and Jean-Frédéric Gerbeau, REO project team, INRIA Paris-Rocquencourt, France, for initiating the SIRAP project. A. Caiazzo, R. Guibert, Y. Boudjemline and I. E. Vignon-Clementel declare that they have no conflict of interest. No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Caiazzo, A., Guibert, R., Boudjemline, Y. et al. Blood Flow Simulations for the Design of Stented Valve Reducer in Enlarged Ventricular Outflow Tracts. Cardiovasc Eng Tech 6, 485–500 (2015). https://doi.org/10.1007/s13239-015-0240-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-015-0240-z