Abstract
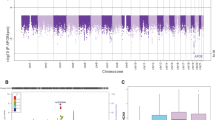
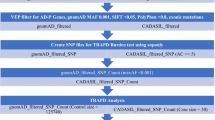
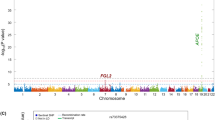
Cerebral amyloid angiopathy (CAA) related intracerebral hemorrhage (ICH) is a devastating form of stroke with no known therapies. Clinical, neuropathological, and genetic studies have suggested both overlap and divergence between the pathogenesis of CAA and the biologically related condition of Alzheimer’s disease (AD). Among the genetic loci associated with AD are APOE and TOMM40, a gene in close proximity to APOE. We investigate here whether variants within TOMM40 are associated with CAA-related ICH and CAA neuropathology. Using cohorts from the Massachusetts General Hospital (MGH) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI), we designed a comparative analysis of high-density SNP genotype data for CAA-related ICH and AD. APOE ε4 was associated with CAA-related ICH and AD, while APOE ε2 was protective in AD but a risk factor for CAA. A total of 14 SNPs within TOMM40 were associated with AD (p < 0.05 after multiple testing correction), but not CAA-related ICH (all p > 0.20); as a result, all AD-associated SNPs within TOMM40 showed heterogeneity of effect in CAA-related ICH (BD p < 0.001). Analysis of CAA neuropathology in the Religious Orders Study (ROS) and Rush Memory and Aging Project (MAP), however, found that neuritic plaque, diffuse plaque burden, and vascular amyloid burden associated with all TOMM40 SNPs (p < 0.02). These results suggest that alterations in TOMM40 can promote vascular as well as plaque amyloid deposition, but not the full pathogenic pathway leading to CAA-related ICH.



Similar content being viewed by others
References
Vinters HV, Gilbery JJ. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–8.
Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–6.
Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60.
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Vinters HV. Cerebral amyloid angiopathy: a microvascular link between parenchymal and vascular dementia? Ann Neurol. 2001;49:691–3.
Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–9.
Van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E genotype and risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342:240–5.
Biffi A, Haplin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–8.
Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28(7):1418–22.
Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–6.
Bekris LM, Yu CE, Bird TD, et al. Genetics of Alzheimer disease. J Geriatr Psychiatry Neurol. 2010;23:213–27.
Revesz T, Holton JL, Lashley T, et al. Genetics and molecular pathologenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009;118:115–30.
Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68(6):934–43.
Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–41.
Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–35.
Hong MG, Alexeyenko A, Lambert JC, et al. Genome-wide pathway analysis implicates intracellular transmembrane protein transport in Alzheimer’s disease. J Hum Genet. 2010;55(10):707–9.
Roses AD. An inherited variable Poly-T repeat genotype in TOMM40 in Alzheimer’s disease. Arch Neurol. 2010;67(5):536–41.
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–31.
Revesz T, Ghiso J, Lashley T, et al. Cerebral amyloid angiopathies: a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003;62(9):885–98.
Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–52.
Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56(4):537–9.
Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–9.
Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Arvanitakis Z, Leurgans SE, Wang Z, et al. Cerebral amyloid angiopathy pathology and cognitive decline in older persons. Ann Neurol. 2011;69:320–7.
Bennett DA, Schneider JA, Buchman AS, et al. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–75.
McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–44.
Bennett DA, Wilson RS, Schneider JA, et al. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60:246–52.
Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18(4 Suppl):S1-2.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (cerad). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86.
Olichney JM, Hansen LA, Hofstetter CR, et al. Cerebral infarction in Alzheimer’s disease is associated with severe amyloid angiopathy and hypertension. Arch Neurol. 1995;52:702–8.
Nakata-Kudo Y, Mizuna T, Yamada K, et al. Microbleeds in Alzheimer disease are more related to cerebral amyloid angiopathy than cerebrovascular disease. Dement Geriatr Cogn Disord. 2006;22:8–14.
Mandybur TI. The incidence of cerebral amyloid angiopahy in Alzheimer’s disease. Neurology. 1975;25:120–6.
Attems J, Jellinger KA. Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology—a pilot study. Acta Neuropathol. 2004;107:83–90.
Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44.
Biffi A, Shulman JM, Jagiella JM, et al. Genetic variation at CR1 increases risk of cerebral amyloid angiopathy. Neurology. 2012;78:1–8.
Biffi A, Anderson CD, Desikan RS, et al. Genetic variation and neuroimaging measures in Alzheimer’s disease. Arch Neruol. 2010;67(6):677–85.
Potkin SG, Guffanti G, Lakatos A, et al. Alzheimer’s Disease Neuroimaging Initiative. Hippocampal atrophy as a quantitative trait in genome-wide association study indentifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4(8):e6501.
Purcell SM, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75.
Breslow NE, Day NE. Statistical methods in cancer research. Volume I—the analysis of case–control studies. IARC Sci Publ. 1980;32:5–338.
Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83:275–81.
Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50(4):961–5.
McCarron MO, Nicoll JA, Stewart J, et al. The apolipoprotein E epsilon2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J Neuropathol Exp Neurol. 1999;58(7):711–8.
Grossman I, Lutz MW, Crenshaw DG, et al. Alzheimer’s disease: diagnostics, prognosis and the road to prevention. EPMA J. 2010;1(2):293–303.
Herculano-Houzel S. Scaling of brain metabolism with a fixed energy budget per neuron: implications for neuronal activity, plasticity and evolution. PLoS One. 2011;6(3):e17514.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–9.
Hansson-Petersen CA, Alikhani N, Behbahani H, et al. The amyloid β-peptide is imported into mitochondrial via the TOM import machinery and localized to mitochondrial cristae. PNAS. 2008;105(35):13145–50.
Purcell SM, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19(1):149–50.
Acknowledgement
The authors thank the participants at MGH and within the ROS and the MAP, the Alzheimer’s Disease Neuroimaging Initiative (ADNI), the Rush Alzheimer’s Disease Center, and the Broad Institute.
Financial Disclosure
The authors declare that they have no conflict of interest.
Funding/Support
This work was supported by the John and Marilyn Keane Stroke Genetics Fund, the Edward and Maybeth Sonn Research Fund, NINDS R01NS059727, the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the National Institutes for Health (NIH) (grant U01 AG024904). The ADNI is funded by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott Laboratories, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation Plc, Genentech Inc, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson Services Inc, Eli Lilly and Company, Medpace Inc, Merck and Co Inc, Novartis International AG, Pfizer Inc, F. Hoffman-La Roche Ltd, and Wyeth Pharmaceuticals, as well as nonprofit partners the Alzheimer’s Association and the Alzheimer’s Drug Discovery Foundation, with participation from the US Food and Drug Administration. Private sector contributions to the ADNI are facilitated by the Foundation for the NIH. The grantee organization is the Northern California Institute for Research and Education Inc, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. The ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angles. This research was also supported by the NIH (grants P30 AG010129 and K01 AG030514) and the Dana Foundation. Drs Biffi and Anderson receive research support from the American Heart Association/Bugher Foundation Centers for Stroke Prevention Research (grant 0775010 N). Dr. Shulman is supported by the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center and Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co., Parkinson’s Study Group, Harvard NeuroDiscovery Center/Massachusetts Alzheimer’s Disease Research Center, and Burroughs Wellcome Fund, Career Award for Medical Scientists. Dr Bennett receives research support from Danone Research B.V., the NIH, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund. Dr DeJager receives research support from Biogen Idec and the NIH. Dr Rosand receives research support from the National Center for Research Resources. Dr Goldstein (grant K23NS059774) receives research support from the National Institute of Health-National Institute of Neurological Disorders and Stroke.
Additional Contributions
We would like to thank Tammy Gills, PhD, and Marcy MacDonald, PhD, for technical assistance in genotyping APOE variants and the investigators participating in the ADNI study, who contributed to the design and implementation of the ADNI and/or provided data but did participate in the analysis or writing of this report.
Author Contributions
Study concept and design: Valant, Rosand and Biffi. Acquisition of data: Valant, Shulman, Devan, Ayres, Schwab, Goldstein, Viswanathan, Greenberg, Bennett, DeJager, Rosand and Biffi. Analysis and interpretation of data: Valant, Keenan, Anderson, Devan, Rosand and Biffi. Drafting of the manuscript: Valant and Biffi. Critical revision of the manuscript for important intellectual comment: Valant, Keenan, Anderson, Shulman, Devan, Ayres, Schwab, Goldstein, Viswanathan, Greenberg, Bennett, De Jager, Rosand and Biffi. Statistical analysis: Keenan, Shulman, Devan and Biffi. Obtained funding: Goldstein, Greenberg, Bennett, De Jager and Rosand. Administrative, technical, and material support: Valant, Ayres and Schwab. Study supervision: Bennett, De Jager, Rosand and Biffi.
Author information
Authors and Affiliations
Consortia
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 92 kb)
Rights and permissions
About this article
Cite this article
Valant, V., Keenan, B.T., Anderson, C.D. et al. TOMM40 in Cerebral Amyloid Angiopathy Related Intracerebral Hemorrhage: Comparative Genetic Analysis with Alzheimer’s Disease. Transl. Stroke Res. 3 (Suppl 1), 102–112 (2012). https://doi.org/10.1007/s12975-012-0161-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0161-1