Abstract
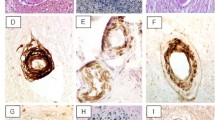
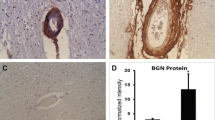
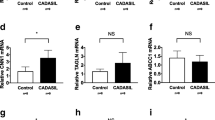
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a genetic disorder hallmarked by ischemic stroke and vascular dementia. Characteristic pathological changes in the vasculature include thickening of small arteries and accumulation of heterogeneous material within the vessel wall. We tested whether endothelial von Willebrand factor (vWF) accumulates in CADASIL vessels and whether exposure of smooth muscle cells to vWF alters the expression of smooth muscle gene expression. Brain sections obtained at autopsy from six North American CADASIL patients were examined using immunohistochemistry for vWF and IgG. Rat aortic smooth muscle cells (A7R5 cells) were tested for binding to infrared tag-labeled vWF. Finally, A7R5 cells were exposed to vWF, and expression of mature smooth muscle marker genes was analyzed by quantitative reverse transcriptase PCR. vWF is expressed in the penetrating arterial walls in all CADASIL samples. IgG, a marker of serum extravasation, was present only in a minority of arterial walls. vWF binds to smooth muscle cells in vitro, and low concentrations of vWF rapidly activate c-Fos, Egr-1, TSP1, and c-Myc while specifically inhibiting RNA encoding smooth muscle actin, calponin, and SM22. These data demonstrate that vWF, likely produced by the endothelium, permeates the vessel wall of CADASIL brains. Exposure of smooth muscle cells to vWF results in reduction of specific RNAs required for normal vascular homeostasis. This is the first report of accumulation of a protein within CADASIL vessels that inhibits vascular gene expression and implicates a role for vWF beyond hemostasis.





Similar content being viewed by others
References
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8(7):643–53.
Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18(22):2730–5.
Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Mohr JP. The natural history of CADASIL: a pooled analysis of previously published cases. Stroke. 1999;30(6):1230–3.
Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O'Sullivan M, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007;69(2):172–9.
Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, Ferrari MD, van Buchem MA, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. 2009;72(2):143–8.
Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, et al. Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL. Stroke. 2007;38(3):923–8.
Jouvent E, Viswanathan A, Mangin JF, O'Sullivan M, Guichard JP, Gschwendtner A, et al. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke. 2007;38(6):1786–90.
Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002;12(3):371–84.
Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14(4):358–64.
Ruchoux MM, Maurage CA. CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56(9):947–64.
Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105(5):597–605.
Low WC, Junna M, Borjesson-Hanson A, Morris CM, Moss TH, Stevens DL, et al. Hereditary multi-infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain. 2007;130(Pt 2):357–67.
Miao Q, Kalimo H, Bogdanovic N, Kostulas K, Borjesson-Hanson A, Viitanen M. Cerebral arteriolar pathology in a 32-year-old patient with CADASIL. Neuropathol Appl Neurobiol. 2006;32(4):455–8.
Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, et al. Arterioles of the lenticular nucleus in CADASIL. Stroke. 2006;37(9):2242–7.
Oide T, Nakayama H, Yanagawa S, Ito N, Ikeda S, Arima K. Extensive loss of arterial medial smooth muscle cells and mural extracellular matrix in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL). Neuropathology. 2008;28(2):132–42.
Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, Bertrand E, Pasennik E, Mendel T, et al. Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL. Immunohistochemical and ultrastructural studies. Folia Neuropathol. 2007;45(4):192–204.
Sadler JE. von Willebrand factor: two sides of a coin. J Thromb Haemost. 2005;3(8):1702–9.
Bosmans JM, Kockx MM, Vrints CJ, Bult H, De Meyer GR, Herman AG. Fibrin(ogen) and von Willebrand factor deposition are associated with intimal thickening after balloon angioplasty of the rabbit carotid artery. Arterioscler Thromb Vasc Biol. 1997;17(4):634–45.
Giddings JC, Banning AP, Ralis H, Lewis MJ. Redistribution of von Willebrand factor in porcine carotid arteries after balloon angioplasty. Arterioscler Thromb Vasc Biol. 1997;17(10):1872–8.
De Meyer GR, Hoylaerts MF, Kockx MM, Yamamoto H, Herman AG, Bult H. Intimal deposition of functional von Willebrand factor in atherogenesis. Arterioscler Thromb Vasc Biol. 1999;19(10):2524–34.
Qin F, Impeduglia T, Schaffer P, Dardik H. Overexpression of von Willebrand factor is an independent risk factor for pathogenesis of intimal hyperplasia: preliminary studies. J Vasc Surg. 2003;37(2):433–9.
Qin F, Dardik H, Pangilinan A, Robinson J, Chuy J, Wengerter K. Remodeling and suppression of intimal hyperplasia of vascular grafts with a distal arteriovenous fistula in a rat model. J Vasc Surg. 2001;34(4):701–6.
Tohgi H, Utsugisawa K, Yoshimura M, Nagane Y, Ukitsu M. Local variation in expression of pro- and antithrombotic factors in vascular endothelium of human autopsy brain. Acta Neuropathol. 1999;98(2):111–8.
Meng H, Zhang X, Lee SJ, Strickland DK, Lawrence DA, Wang MM. Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. J Biol Chem. 2010;285(30):23047–55.
Meng H, Zhang X, Hankenson KD, Wang MM. Thrombospondin 2 potentiates notch3/jagged1 signaling. J Biol Chem. 2009;284(12):7866–74.
Alafuzoff I, Adolfsson R, Bucht G, Winblad B. Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci. 1983;60(3):465–72.
Alafuzoff I, Adolfsson R, Grundke-Iqbal I, Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. An immunocytochemical study. Acta Neuropathol. 1987;73(2):160–6.
Cui TX, Piwien-Pilipuk G, Huo JS, Kaplani J, Kwok R, Schwartz J. Endogenous CCAAT/enhancer binding protein beta and p300 are both regulated by growth hormone to mediate transcriptional activation. Mol Endocrinol. 2005;19(8):2175–86.
Bergmann M, Ebke M, Yuan Y, Bruck W, Mugler M, Schwendemann G. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): a morphological study of a German family. Acta Neuropathol. 1996;92(4):341–50.
Gray F, Robert F, Labrecque R, Chretien F, Baudrimont M, Fallet-Bianco C, et al. Autosomal dominant arteriopathic leuko-encephalopathy and Alzheimer's disease. Neuropathol Appl Neurobiol. 1994;20(1):22–30.
Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol. 1995;89(6):500–12.
Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120(2):433–45.
Rand JH, Wu XX, Potter BJ, Uson RR, Gordon RE. Co-localization of von Willebrand factor and type VI collagen in human vascular subendothelium. Am J Pathol. 1993;142(3):843–50.
Brulin P, Godfraind C, Leteurtre E, Ruchoux MM. Morphometric analysis of ultrastructural vascular changes in CADASIL: analysis of 50 skin biopsy specimens and pathogenic implications. Acta Neuropathol. 2002;104(3):241–8. doi:10.1007/s00401-002-0530-z.
Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, et al. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol. 2003;162(1):329–42.
Kageyama S, Yamamoto H, Yoshimoto R. Anti-human von willebrand factor monoclonal antibody AJvW-2 prevents thrombus deposition and neointima formation after balloon injury in guinea pigs. Arterioscler Thromb Vasc Biol. 2000;20(10):2303–8.
Methia N, Andre P, Denis CV, Economopoulos M, Wagner DD. Localized reduction of atherosclerosis in von Willebrand factor-deficient mice. Blood. 2001;98(5):1424–8.
Bongers TN, de Maat MP, van Goor ML, Bhagwanbali V, van Vliet HH, Gomez Garcia EB, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37(11):2672–7.
Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100(7):736–42.
Qizilbash N, Duffy S, Prentice CR, Boothby M, Warlow C. von Willebrand factor and risk of ischemic stroke. Neurology. 1997;49(6):1552–6.
Acknowledgments
We thank the patients and families who donated tissues for this study. We also acknowledge the generous resources provided by the University of Michigan Alzheimer's Disease Research Center (funded by NIH P50 AG008761-20-SI), who provided control tissues. Other human tissues were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, contract HHSN275200900011C, ref. no. NO1-HD-9-0011. Shannon Dunn contributed to c-Fos luciferase studies.
Sources of Funding
This study was supported by grants NS054724 and NS062816 from NIH-NINDS and VA Merit award 5I01BX000375.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Meng, H., Blaivas, M. et al. von Willebrand Factor Permeates Small Vessels in CADASIL and Inhibits Smooth Muscle Gene Expression. Transl. Stroke Res. 3, 138–145 (2012). https://doi.org/10.1007/s12975-011-0112-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-011-0112-2