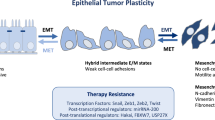
Abstract
The epithelial-mesenchymal transition (EMT) is a complex series of cellular reprogramming events that culminates in the loss of epithelial characteristics and the de novo acquisition of a mesenchymal phenotype. During embryonic development, EMT imparts the plasticity and migratory capabilities that enable the extensive cell movements underlying gastrulation and organogenesis. In breast cancer, aberrant activation of EMT is associated with the highly aggressive basal-like, metaplastic, and claudin-low tumor subtypes and confers increased cell survival, stem-like properties, and migratory and invasive capabilities, thus promoting cancer cell dissemination and metastasis. As the EMT program is primarily enlisted during early embryonic development, and as EMT orchestrators are mostly dormant in normal adult tissues, targeting EMT drivers may have minimal side effects. Therefore, a better understanding of EMT at the molecular level is likely to yield new insight into the mechanisms of breast cancer progression, enable earlier detection of metastases, and ultimately suggest new avenues for therapeutic intervention.
Similar content being viewed by others
References and Recommended Reading
Hollier BG, Evans K, Mani SA: The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia 2009, 14:29–43.
Polyak K, Weinberg RA: Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009, 9:265–273.
Thompson EW, Newgreen DF, Tarin D: Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res 2005, 65:5991–5995; discussion 5995.
Santisteban M, Reiman JM, Asiedu MK, et al.: Immuneinduced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res 2009, 69:2887–2895.
Blick T, Widodo E, Hugo H, et al.: Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis 2008, 25:629–642.
Sarrio D, Rodriguez-Pinilla SM, Hardisson D, et al.: Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 2008, 68:989–997.
Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, et al.: Changes in cytoskeletal protein composition indicative of an epithelial-mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res 2005, 11:8006–8014.
Berx G, Van Roy F: The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 2001, 3:289–293.
Baranwal S, Alahari SK: Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun 2009, 384:6–11.
Onder TT, Gupta PB, Mani SA, et al.: Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008, 68:3645–3654.
Yang J, Mani SA, Donaher JL, et al.: Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117:927–939.
Sarrio D, Palacios J, Hergueta-Redondo M, et al.: Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 2009, 9:74.
Vandewalle C, Comijn J, De Craene B, et al.: SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res 2005, 33:6566–6578.
Aigner K, Dampier B, Descovich L, et al.: The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 2007, 26:6979–6988.
Hazan RB, Phillips GR, Qiao RF, et al.: Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 2000, 148:779–790.
Pieper FR, Van de Klundert FA, Raats JM, et al.: Regulation of vimentin expression in cultured epithelial cells. Eur J Biochem 1992, 210:509–519.
Gilles C, Polette M, Mestdagt M, et al.: Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res 2003, 63:2658–2664.
Dandachi N, Hauser-Kronberger C, More E, et al.: Coexpression of tenascin-C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 2001, 193:181–189.
Polette M, Mestdagt M, Bindels S, et al.: Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 2007, 185:61–65.
Bindels S, Mestdagt M, Vandewalle C, et al.: Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene 2006, 25:4975–4985.
Xue C, Plieth D, Venkov C, et al.: The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res 2003, 63:3386–3394.
Blanco MJ, Moreno-Bueno G, Sarrio D, et al.: Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 2002, 21:3241–3246.
Hartwell KA, Muir B, Reinhardt F, et al.: The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A 2006, 103:18969–18974.
Mani SA, Yang J, Brooks M, et al.: Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A 2007, 104:10069–10074.
Moody SE, Perez D, Pan TC, et al.: The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 2005, 8:197–209.
Martin TA, Goyal A, Watkins G, Jiang WG: Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol 2005, 12:488–496.
Come C, Magnino F, Bibeau F, et al.: Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res 2006, 12:5395–5402.
Laffin B, Wellberg E, Kwak HI, et al.: Loss of singleminded- 2s in the mouse mammary gland induces an epithelial-mesenchymal transition associated with up-regulation of slug and matrix metalloprotease 2. Mol Cell Biol 2008, 28:1936–1946.
Lester RD, Jo M, Montel V, et al.: uPAR induces epithelialmesenchymal transition in hypoxic breast cancer cells. J Cell Biol 2007, 178:425–436.
Storci G, Sansone P, Trere D, et al.: The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J Pathol 2008, 214:25–37.
Wu Y, Deng J, Rychahou PG, et al.: Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 2009, 15:416–428.
Sullivan NJ, Sasser AK, Axel AE, et al.: Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009 (in press).
Petersen OW, Nielsen HL, Gudjonsson T, et al.: Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol 2003, 162:391–402.
Mani SA, Guo W, Liao MJ, et al.: The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133:704–715.
Al-Hajj M, Wicha MS, Benito-Hernandez A, et al.: Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003, 100:3983–3988.
Morel AP, Lievre M, Thomas C, et al.: Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008, 3:e2888.
Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, et al.: Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res 2009, 69:4116–4124.
DiMeo TA, Anderson K, Phadke P, et al.: A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basallike breast cancer. Cancer Res 2009, 69:5364–5373.
Stasinopoulos IA, Mironchik Y, Raman A, et al.: HOXA5-twist interaction alters p53 homeostasis in breast cancer cells. J Biol Chem 2005, 280:2294–2299.
Ansieau S, Bastid J, Doreau A, et al.: Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 2008, 14:79–89.
Li QQ, Xu JD, Wang WJ, et al.: Twist1-mediated adriamycininduced epithelial-mesenchymal transition relates to multidrug resistance and invasive potential in breast cancer cells. Clin Cancer Res 2009, 15:2657–2665.
Kajita M, McClinic KN, Wade PA: Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol 2004, 24:7559–7566.
Smit MA, Geiger TR, Song JY, et al.: A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 2009, 29:3722–3737.
Evdokimova V, Tognon C, Ng T, et al.: Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell 2009, 15:402–415.
Li X, Lewis MT, Huang J, et al.: Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 2008, 100:672–679.
Creighton CJ, Li X, Landis M, et al.: Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009 (in press).
Gregory PA, Bert AG, Paterson EL, et al.: The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008, 10:593–601.
Park SM, Gaur AB, Lengyel E, Peter ME: The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008, 22:894–907.
Bracken CP, Gregory PA, Kolesnikoff N, et al.: A doublenegative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008, 68:7846–7854.
Burk U, Schubert J, Wellner U, et al.: A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 2008, 9:582–589.
Shimono Y, Zabala M, Cho RW, et al.: Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138:592–603.
Ma L, Teruya-Feldstein J, Weinberg RA: Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007, 449:682–688.
Huang Q, Gumireddy K, Schrier M, et al.: The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol 2008, 10:202–210.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sphyris, N., Mani, S.A. The importance of the epithelial-mesenchymal transition in breast cancer. Curr Breast Cancer Rep 1, 229–237 (2009). https://doi.org/10.1007/s12609-009-0032-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-009-0032-2