Abstract
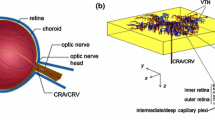
To quantitatively assess the arteriovenous distribution of hemodynamic parameters throughout the microvascular network of the human retina, we constructed a retinal microcirculatory model consisting of a dichotomous symmetric branching system. This system is characterized by a diameter exponent of 2.85, instead of 3 as dictated by Murray’s law, except for the capillary networks. The value of 2.85 was the sum of a fractal dimension (1.70) and a branch exponent (1.15) of the retinal vasculature. Following the feeding artery (central retinal artery), each bifurcation was recursively developed at a distance of an individual branch length [L(r) = 7.4r 1.15] by a centrifugal scheme. The venular tree was formed in the same way. Using this model, we evaluated hemodynamic parameters, including blood pressure, blood flow, blood velocity, shear rate, and shear stress, within the retinal microcirculatory network as a function of vessel diameter. The arteriovenous distributions of blood pressure and velocity in the simulation were consistent with in vivo measurements in the human retina and other vascular beds of small animals. We therefore conclude that the current theoretical model was useful for quantifying hemodynamics as a function of vessel diameter within the retinal microvascular network.




Similar content being viewed by others
References
Murray CD. The physiological principle of minimum work I. The vascular system and the cost of blood volume. Proc Natl Acad Sci USA. 1926;12:207–14.
Sherman TF. On concerning large vessels to small: the meaning of Murray’s law. J Gen Physiol. 1981;78:431–53.
Kamiya A, Bukhari R, Togawa T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull Math Biol. 1984;46:127–37.
Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine artery. Am J Physiol. 1980;239:H14–21.
Kamiya A, Takahashi T. Quantitative assessments of morphological and functional properties of biological trees based on their fractal nature. J Appl Physiol. 2007;102:2315–23.
Fåhraeus R, Lindqvist T. The viscosity of the blood in narrow capillary tubes. Am J Physiol. 1931;96:562–8.
Wong TY, Klein R, Klein BEK, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80.
Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–48.
Nagaoka T, Yoshida A. Noninvasive evaluation of wall shear stress on retinal microcirculation in humans. Invest Opthalmol Vis Sci. 2006;47:1113–9.
Suwa N, Takahashi T. Morphological and morphometrical analysis of circulation in hypertension and ischemic kidney. Munich: Urban & Schwarzenberg; 1971.
Mandelbrot BB. The fractal geometry of nature. New York: Freeman; 1983.
Bassingthwaite JB, Liebovitch LS, West BJ. Fractal physiology. Oxford: Oxford University Press; 1994.
Masters BR. Fractal analysis of normal human retinal blood vessels. Fractals. 1994;2:103–10.
Amemiya T. Retinal and choroidal vascular changes and systemic diseases in rats. Tokyo: Springer; 2003.
Haynes RH. Physical basis of the dependence of blood viscosity on tube radius. Am J Physiol. 1960;198:1193–200.
Chien S, Usami S, Skalak R. Blood flow in small tubes. In: Renkin EM, Michel CC, editors. Handbook of physiology. The cardiovascular system, microcirculation. Bethesda: American Physiological Society; 1984. p. 217–49.
Bill A. Circulation of the eye. In: Renkin EM, Michel CC, editors. Handbook of physiology. The cardiovascular system, microcirculation. Bethesda: American Physiological Society; 1984. p. 1001–34.
Glucksberg MR, Dunn R. Direct measurement of retinal microvascular pressures in the live, anesthetized cat. Microvasc Res. 1993;45:158–65.
Zamir M, Medeiros JA. Arterial branching in man and monkey. J Gen Physiol. 1982;79:353–60.
Rossitti S, Frisén L. Remodelling of the retinal arterioles in descending optic atrophy follows the principle of minimum work. Acta Physiol Scand. 1994;152:333–40.
Skalak R, Özkaya N. Biofluid mechanics. Annu Rev Fluid Mech. 1989;21:167–204.
Fung YC. Biomechanics: circulation. New York: Springer; 1996.
Riva CE, Grunwald JE, Sinclair SH, Petring BL. Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci. 1985;26:1124–32.
Fronek K, Zweifach BW. Microvascular pressure distribution in skeletal muscle and the effect of vasodilation. Am J Physiol. 1975;228:791–6.
Zweifach BW, Lipowsky HH. Quantitative studies of microvascular structure and function III. Microvascular hemodynamics of cat mesentery and rabbit omentum. Circ Res. 1977;41:380–90.
Zweifach BW, Kovalcheck S, DeLano F, Chen P. Micropressure-flow relationships in a skeletal muscle of spontaneously hypertensive rats. Hypertension. 1981;3:601–14.
Zweifach BW. Quantitative studies of microvascular structure and function I. Analysis of pressure distribution in the terminal vascular bed in cat mesentery. Circ Res. 1974;34:843–57.
Takahashi T, Okada A, Saitoh T, Hayano J, Miyamoto Y. Difference in human cardiovascular response between upright and supine recovery from upright cycle exercise. Eur J Appl Physiol. 2000;81:233–9.
Landis EM, Pappenheimer JR. Exchange of substances through the capillary walls. In: Hamilton WF, editor. Handbook of physiology. Circulation. Washington D.C.: American Physiological Society; 1963. p. 961–1034.
Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, et al. Nitric oxide regulation retinal vascular tone in humans. Am J Physiol. 2003;285:H631–6.
Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–25.
Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol. 1992;263:H641–6.
Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–5.
Van Beek JHGM, Roger SA, Bassingthwaighte JB. Regional myocardial flow heterogeneity explained with fractal networks. Am J Physiol. 1989;257:H1670–80.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takahashi, T., Nagaoka, T., Yanagida, H. et al. A mathematical model for the distribution of hemodynamic parameters in the human retinal microvascular network. J Biorheol 23, 77–86 (2009). https://doi.org/10.1007/s12573-009-0012-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12573-009-0012-1