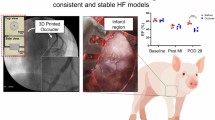
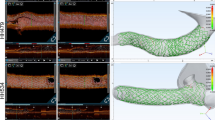
Abstract
Reliable, closed-chest methods for creating large animal models of acute myocardial hypoperfusion are limited. We demonstrated the feasibility and efficacy of using magnetic resonance (MR)–compatible 3D-printed coronary implants for establishing swine models of myocardial hypoperfusion. We designed, manufactured, and percutaneously deployed implants in 13 swine to selectively create focal coronary stenosis. To test the efficacy of the implants to cause hypoperfusion or ischemia in the perfused territory, we evaluated regional wall motion, myocardial perfusion, and infarction using MR imaging. The overall swine survival rate was 85% (11 of 13). The implant retrieval rate was 92% (12 of 13). Fluoroscopic angiography confirmed focal stenosis. Cine and perfusion MRI showed regional wall motion abnormalities and inducible ischemia, respectively. Late gadolinium enhancement and histopathology showed no myocardial infarction. Our minimally invasive technique has promising applications for validation of new diagnostic methods in cardiac MR.

Our new minimally invasive, percutaneous method for creating swine models of acute focal coronary stenosis can be used for magnetic resonance imaging studies of myocardial ischemia. Comparable to existing methods in its efficacy and reliability, this rapid prototyping technique will allow researchers to more easily conduct translational cardiac imaging studies of coronary artery disease in large animal models.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FOV:
-
Field of view
- IHD:
-
Ischemic heart disease
- MRI:
-
Magnetic resonance imaging
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
- TTC:
-
Triphenyltetrazolium chloride
References
Keeran, K. J., Jeffries, K. R., Zetts, A. D., Taylor, J., Kozlov, S., & Hunt, T. J. (2017). A chronic cardiac ischemia model in swine using an ameroid constrictor. Journal of Visualized Experiments, 128. https://doi.org/10.3791/56190.
Litvak, J., Siderides, L. E., & Vineberg, A. M. (1957). The experimental production of coronary artery insufficiency and occlusion. American Heart Journal, 53(4), 505–518. https://doi.org/10.1016/0002-8703(57)90359-9.
Hughes, G. C., Post, M. J., Simons, M., & Annex, B. H. (2015). Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. Journal of Applied Physiology, 94(5), 1689–1701. https://doi.org/10.1152/japplphysiol.00465.2002.
Robich, M. P., Chu, L. M., Burgess, T. A., Feng, J., Han, Y., Nezafat, R., et al. (2012). Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. Journal of the American College of Surgeons, 215(5), 681–689. https://doi.org/10.1016/j.jamcollsurg.2012.06.417.
Robich, M. P., Osipov, R. M., Nezafat, R., Feng, J., Clements, R. T., Bianchi, C., et al. (2010). Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation, 122(11 SUPPL. 1), S142–S149. https://doi.org/10.1161/CIRCULATIONAHA.109.920132.
Sabe, A. A., Elmadhun, N. Y., Robich, M. P., Dalal, R. S., & Sellke, F. W. (2013). Does resveratrol improve insulin signaling in chronically ischemic myocardium? Journal of Surgical Research, 183(2), 531–536. https://doi.org/10.1016/j.jss.2013.03.004.
Kraitchman, D., Bluemke, D., Chin, B., & Heldman, A. (2000). A minimally invasive method for creating coronary stenosis in a swine model for MRI and SPECT imaging. Investigative Radiology, 35(7), 445–451. https://doi.org/10.1097/00004424-200007000-00008.
Gerber, B. L., Boston, R. C., Kraitchman, D. L., Chin, B. B., Lima, J. A. C., Bluemke, D. A., & Heldman, A. W. (2007). Single-vessel coronary artery stenosis: myocardial perfusion imaging with Gadomer-17 first-pass MR imaging in a swine model of comparison with gadopentetate dimeglumine. Radiology, 225(1), 104–112. https://doi.org/10.1148/radiol.2251011377.
Wu, M., Bogaert, J., D’Hooge, J., Sipido, K., Maes, F., Dymarkowski, S., et al. (2010). Closed-chest animal model of chronic coronary artery stenosis. Assessment with magnetic resonance imaging. International Journal of Cardiovascular Imaging, 26(3), 299–308. https://doi.org/10.1007/s10554-009-9551-1.
Foin, N., Sen, S., Petraco, R., Nijjer, S., Torii, R., Kousera, C., et al. (2013). Method for percutaneously introducing, and removing, anatomical stenosis of predetermined severity in vivo: the “stenotic stent.”. Journal of Cardiovascular Translational Research, 6(4), 640–648. https://doi.org/10.1007/s12265-013-9476-x.
Rissanen, T. T., Nurro, J., Halonen, P. J., Tarkia, M., Saraste, A., Rannankari, M., et al. (2013). The bottleneck stent model for chronic myocardial ischemia and heart failure in pigs. American Journal of Physiology - Heart and Circulatory Physiology, 305(9), 1297–1308. https://doi.org/10.1152/ajpheart.00561.2013.
Krombach, G. A., Kinzel, S., Mahnken, A. H., Günther, R. W., & Buecker, A. (2005). Minimally invasive close-chest method for creating reperfused or occlusive myocardial infarction in swine. Investigative Radiology, 40(1), 14–18.
Nguyen, K.-L., Shao, J., Ghodrati, V. K., Ajijola, O. A., Dharmakumar, R., Finn, J. P., & Hu, P. (2019). Ferumoxytol-enhanced CMR for vasodilator stress testing: a feasibility study. JACC: Cardiovascular Imaging, 12(8), 1582–1584. https://doi.org/10.1016/j.jcmg.2019.01.024.
Giannopoulos, A. A., Mitsouras, D., Yoo, S. J., Liu, P. P., Chatzizisis, Y. S., & Rybicki, F. J. (2016). Applications of 3D printing in cardiovascular diseases. Nature Reviews Cardiology, 13(12), 701–718. https://doi.org/10.1038/nrcardio.2016.170.
de Oliveira-Santos, M., Oliveira-Santos, E., Gonçalves, L., & Silva Marques, J. (2019). Cardiovascular three-dimensional printing in non-congenital percutaneous interventions. Heart, Lung & Circulation, 28(10), 1525–1534. https://doi.org/10.1016/j.hlc.2019.04.020.
Forte, M. N. V., Hussain, T., Roest, A., Gomez, G., Jongbloed, M., Simpson, J., et al. (2019). Living the heart in three dimensions: applications of 3D printing in CHD. Cardiology in the Young, 29(6), 733–743. https://doi.org/10.1017/S1047951119000398.
Alonzo, M., AnilKumar, S., Roman, B., Tasnim, N., & Joddar, B. (2019). 3D bioprinting of cardiac tissue and cardiac stem cell therapy. Translational Research, 211, 64–83. https://doi.org/10.1016/j.trsl.2019.04.004.
Lee, W., Hong, Y., & Dai, G. (2019). 3D bioprinting of vascular conduits for pediatric congenital heart repairs. Translational Research, 211, 35–45. https://doi.org/10.1016/j.trsl.2019.03.007.
Hollowed, J. J., Colbert, C. M., Currier, J. W., & Nguyen, K.-L. (2020). Novel percutaneous approach for deployment of 3D printed coronary stenosis implants in swine models of ischemic heart disease. Journal of Visualized Experiments, e60729. https://doi.org/10.3791/60729.
Shaw, L. J., Berman, D. S., Maron, D. J., Mancini, G. B. J., Hayes, S. W., Hartigan, P. M., et al. (2008). Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation, 117(10), 1283–1291. https://doi.org/10.1161/CIRCULATIONAHA.107.743963.
Liu, A., Wijesurendra, R. S., Liu, J. M., Greiser, A., Jerosch-Herold, M., Forfar, J. C., et al. (2018). Gadolinium-free cardiac MR stress T1-mapping to distinguish epicardial from microvascular coronary disease. Journal of the American College of Cardiology, 71(9), 957–968. https://doi.org/10.1016/j.jacc.2017.11.071.
Husso, M., Nissi, M. J., Kuivanen, A., Halonen, P., Tarkia, M., Teuho, J., et al. (2019). Quantification of porcine myocardial perfusion with modified dual bolus MRI-a prospective study with a PET reference. BMC Medical Imaging, 19(1), 1–11. https://doi.org/10.1186/s12880-019-0359-8.
Fishbein, M. C., Meerbaum, S., Rit, J., Lando, U., Kanmatsuse, K., Mercier, J. C., et al. (1981). Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. American Heart Journal, 101(5), 593–600. https://doi.org/10.1016/0002-8703(81)90226-X.
Fieno, D. S., Kim, R. J., Chen, E. L., Lomasney, J. W., Klocke, F. J., & Judd, R. M. (2000). Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. Journal of the American College of Cardiology, 36(6), 1985–1991. https://doi.org/10.1016/S0735-1097(00)00958-X.
Lindsey, M. L., Bolli, R., Canty, J. M., Du, X.-J., Frangogiannis, N. G., Frantz, S., et al. (2018). Guidelines for experimental models of myocardial ischemia and infarction. American journal of physiology. Heart and Circulatory Physiology, 314(4), H812–H838. https://doi.org/10.1152/ajpheart.00335.2017.
Mahrholdt, H., Wagner, A., Judd, R. M., Sechtem, U., & Kim, R. J. (2005). Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. European Heart Journal. https://doi.org/10.1093/eurheartj/ehi258.
Liao, J., Huang, W., & Liu, G. (2016). Animal models of coronary heart disease. Journal of Biomedical Research, 30(1), 3–10. https://doi.org/10.7555/jbr.30.20150051.
Tanimoto, A., Kawaguchi, H., Yoshida, H., Misumi, K., Fujiki, M., Miyoshi, N., et al. (2011). Microminipig, a non-rodent experimental animal optimized for life science research: novel atherosclerosis model induced by high fat and cholesterol diet. Journal of Pharmacological Sciences, 115(2), 115–121. https://doi.org/10.1254/jphs.10r17fm.
Adams, G. J., Baltazar, U., Karmonik, C., Bordelon, C., Lin, P. H., Bush, R. L., et al. (2005). Comparison of 15 different stents in superficial femoral arteries by high resolution MRI ex vivo and in vivo. Journal of Magnetic Resonance Imaging, 22(1), 125–135. https://doi.org/10.1002/jmri.20359.
Schuster, A., Sinclair, M., Zarinabad, N., Ishida, M., Van Den Wijngaard, J. P. H. M., Paul, M., et al. (2015). A quantitative high resolution voxel-wise assessment of myocardial blood flow from contrast-enhanced first-pass magnetic resonance perfusion imaging: microsphere validation in a magnetic resonance compatible free beating explanted pig heart model. European Heart Journal Cardiovascular Imaging, 16(10), 1082–1092. https://doi.org/10.1093/ehjci/jev023.
Elzinga, W. E. (1969). Ameroid constrictor: uniform closure rates and a calibration procedure. Journal of Applied Physiology, 27(3), 419–421. https://doi.org/10.1152/jappl.1969.27.3.419.
Inou, T., Tomoike, T., Watanabe, K., Kikuchi, Y., Mizukami, M., Kurozumi, T., & Nakamura, M. (1980). A newly developed X-ray transparent ameroid constrictor for study on progression of gradual coronary stenosis. Basic Research in Cardiology, 75(4), 537–543. https://doi.org/10.1007/BF01907835.
Guo, M., Liu, J., Guo, F., Shi, J., Wang, C., Bible, P. W., et al. (2018). Panax quinquefolium saponins attenuate myocardial dysfunction induced by chronic ischemia. Cellular Physiology and Biochemistry, 49(4), 1277–1288. https://doi.org/10.1159/000493407.
Biran, R., & Pond, D. (2017). Heparin coatings for improving blood compatibility of medical devices. Advanced Drug Delivery Reviews, 112, 12–23. https://doi.org/10.1016/j.addr.2016.12.002.
Bressi, E., Mangiacapra, F., Morisco, C., Barbato, E., & Sticchi, A. (2018). Fractional flow reserve (FFR) as a guide to treat coronary artery disease. Expert Review of Cardiovascular Therapy, 16(7), 465–477. https://doi.org/10.1080/14779072.2018.1489236.
Hecht, H. S., Narula, J., & Fearon, W. F. (2016). Fractional flow reserve and coronary computed tomographic angiography: a review and critical analysis. Circulation Research, 119(2), 300–316. https://doi.org/10.1161/CIRCRESAHA.116.307914.
Acknowledgments
We thank staff members at the UCLA Lux Lab, the UCLA Translational Research Imaging Center (TRIC), and the Division of Laboratory Animal Medicine at UCLA for their assistance.
Funding
This work is supported by American Heart Association Transformational Award 18TPA34170049 and pilot funding from the Department of Radiology and Medicine at David Geffen School of Medicine at UCLA. K.L.N. is supported by funding from the American Heart Association (18TPA34170049), Veterans Health Administration (CX001901), and NIH (HL137562). O.A.A. is supported by NIH (HL142045). P.H. is supported by funding from NIH (HL127153), the American Heart Association (18TPA34170049), and the Veterans Health Administration (CX001901). R.D is supported by funding from NIH (HL133407, HL136578, and HL147133).
Author information
Authors and Affiliations
Contributions
Conception and design (KLN, PH, CMC), analysis and interpretation (CMC, KLN), data collection (CMC, KLN, JS, JHH, JWC, OAA, SMD, GAF), drafting the article (CMC, KLN), critical revision of the article (all authors), final approval of the article (all authors), overall responsibility (KLN)
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Human Studies
No human studies were carried out by the authors for this article.
Animal Studies
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by our Institutional Animal Care and Use Committee (protocol no. 015-03D). With permission, S&S Farms supplied swine models purposely bred for biomedical research. S&S Farms Domestic pigs are a Yorkshire/Landrace hybrid originally derived from a specific pathogen-free herd.
Additional information
Associate Editor Adrian Chester oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical Relevance
Reliability of closed-chest techniques to create swine models of acute coronary stenosis remains limited. This work demonstrates the feasibility and efficacy of a rapid prototyping method using 3D-printed, heparin-coated, coronary stenosis implants to percutaneously create swine models of myocardial hypoperfusion and ischemia, which can be used to evaluate novel diagnostic MRI methods for ischemic coronary heart disease.
Rights and permissions
About this article
Cite this article
Colbert, C.M., Shao, J., Hollowed, J.J. et al. 3D-Printed Coronary Implants Are Effective for Percutaneous Creation of Swine Models with Focal Coronary Stenosis. J. of Cardiovasc. Trans. Res. 13, 1033–1043 (2020). https://doi.org/10.1007/s12265-020-10018-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10018-3