Abstract
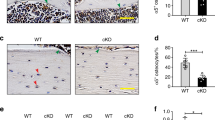
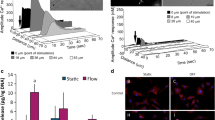
Since integrins were first described as cell adhesion receptors over two decades ago, our understanding of their binding specificity and functional capacity has evolved dramatically. A number of in vitro cell culture experiments have suggested that integrins may play a role in the response of bone cells to mechanical stimuli. To determine whether the loss of integrins in bone cells affects mechanical adaptation in vivo, we used an ulnar loading model in mice with an osteocyte-specific β1 integrin deficiency. Using a Cre-loxP strategy in which Cre was driven by the 2.3 kb ColI(α1) promoter, the β1 integrin subunit was deleted from cortical osteocytes in mature (16 week old) mice. While there was no observable skeletal phenotype as a result of β1 integrin deletion, we found that conditional knockout mice exhibited a significant reduction in bone formation rates at the ulnar midshaft in response to three consecutive days of cyclic loading compared to floxed control mice. Further, there was a greater increase in periosteal expansion in control vs. conditional knockout mice in response to loading. While there are likely multiple signaling pathways involved in the cellular response to physical stimuli, our results suggest that β1 integrins play a role in mechanically induced bone formation.




Similar content being viewed by others
Abbreviations
- cKO:
-
Conditional knockout
- ColI(α1):
-
Type I collagen α1(I)
- MAR:
-
Mineral apposition rate
- MS/BS:
-
Mineralizing surface
- BFR:
-
Bone formation rate
References
Aguirre, J. I., L. I. Plotkin, S. A. Stewart, R. S. Weinstein, A. M. Parfitt, S. C. Manolagas, and T. Bellido. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21(4):605–615, 2006.
Arcangeli, A., and A. Becchetti. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 16(12):631–639, 2006.
Boutahar, N., A. Guignandon, L. Vico, and M. H. Lafage-Proust. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in erk activation. J. Biol. Chem. 279(29):30588–30599, 2004.
Brakebusch, C., and R. Fassler. Beta 1 integrin function in vivo: adhesion, migration and more. Cancer Metastasis Rev. 24(3):403–411, 2005.
Dacic, S., I. Kalajzic, D. Visnjic, A. C. Lichtler, and D. W. Rowe. Col1a1-driven transgenic markers of osteoblast lineage progression. J. Bone Miner. Res. 16(7):1228–1236, 2001.
Dacquin, R., M. Starbuck, T. Schinke, and G. Karsenty. Mouse alpha1(i)-collagen promoter is the best known promoter to drive efficient cre recombinase expression in osteoblast. Dev. Dyn. 224(2):245–251, 2002.
Globus, R. K., D. Amblard, Y. Nishimura, U. T. Iwaniec, J. B. Kim, E. A. Almeida, C. D. Damsky, T. J. Wronski, and M. C. van der Meulen. Skeletal phenotype of growing transgenic mice that express a function-perturbing form of beta1 integrin in osteoblasts. Calcif. Tissue Int. 76(1):39–49, 2005.
Gronthos, S., K. Stewart, S. E. Graves, S. Hay, and P. J. Simmons. Integrin expression and function on human osteoblast-like cells. J. Bone Miner. Res. 12(8):1189–1197, 1997.
Grzesik, W. J., and P. G. Robey. Bone matrix rgd glycoproteins: Immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J. Bone Miner. Res. 9(4):487–496, 1994.
Gullberg, D. E., and E. Lundgren-Akerlund. Collagen-binding i domain integrins—what do they do? Prog. Histochem. Cytochem. 37(1):3–54, 2002.
Hirai, F., S. Nakayamada, Y. Okada, K. Saito, H. Kurose, A. Mogami, and Y. Tanaka. Small gtpase rho signaling is involved in beta1 integrin-mediated up-regulation of intercellular adhesion molecule 1 and receptor activator of nuclear factor kappab ligand on osteoblasts and osteoclast maturation. Biochem. Biophys. Res. Commun. 356(1):279–285, 2007.
Horton, M. A., H. M. Massey, N. Rosenberg, B. Nicholls, U. Seligsohn, and A. M. Flanagan. Upregulation of osteoclast alpha2beta1 integrin compensates for lack of alphavbeta3 vitronectin receptor in iraqi-jewish-type glanzmann thrombasthenia. Br. J. Haematol. 122(6):950–957, 2003.
Hsieh, Y. F., T. Wang, and C. H. Turner. Viscoelastic response of the rat loading model: Implications for studies of strain-adaptive bone formation. Bone 25(3):379–382, 1999.
Huang, Z., K. Shimazu, N. H. Woo, K. Zang, U. Muller, B. Lu, and L. F. Reichardt. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J. Neurosci. 26(43):11208–11219, 2006.
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687, 2002.
Imai, T., M. Jiang, P. Chambon, and D. Metzger. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid x receptor alpha mediated by a tamoxifen-inducible chimeric cre recombinase (cre-ert2) in adipocytes. Proc. Natl Acad. Sci. USA 98(1):224–228, 2001.
Iwaniec, U. T., T. J. Wronski, D. Amblard, Y. Nishimura, M. C. van der Meulen, C. E. Wade, M. A. Bourgeois, C. D. Damsky, and R. K. Globus. Effects of disrupted beta1-integrin function on the skeletal response to short-term hindlimb unloading in mice. J. Appl. Physiol. 98(2):690–696, 2005.
Katsumi, A., A. W. Orr, E. Tzima, and M. A. Schwartz. Integrins in mechanotransduction. J. Biol. Chem. 279(13):12001–12004, 2004.
Leucht, P., J. B. Kim, J. A. Currey, J. Brunski, and J. A. Helms. Fak-mediated mechanotransduction in skeletal regeneration. PLoS ONE 2(4):e390, 2007.
McNamara, L. M., R. J. Majeska, S. Weinbaum, V. Friedrich, and M. B. Schaffler. Attachment of osteocyte cell processes to the bone matrix. Anat. Rec. Hoboken 292(3):355–363, 2009.
Mercurio, A. M. Lessons from the alpha2 integrin knockout mouse. Am. J. Pathol. 161(1):3–6, 2002.
Nakayamada, S., Y. Okada, K. Saito, M. Tamura, and Y. Tanaka. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappab ligand on osteoblasts and osteoclast maturation. J. Biol. Chem. 278(46):45368–45374, 2003.
Parfitt, A. M., M. K. Drezner, F. H. Glorieux, J. A. Kanis, H. Malluche, P. J. Meunier, S. M. Ott, and R. R. Recker. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the asbmr histomorphometry nomenclature committee. J. Bone Miner. Res. 2(6):595–610, 1987.
Phillips, J. A., E. A. Almeida, E. L. Hill, J. I. Aguirre, M. F. Rivera, I. Nachbandi, T. J. Wronski, M. C. van der Meulen, and R. K. Globus. Role for beta1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 27:609–618, 2008.
Ponik, S. M., and F. M. Pavalko. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of cox-2 and pge2 release in mc3t3-e1 osteoblasts. J. Appl. Physiol. 97(1):135–142, 2004.
Potocnik, A. J., C. Brakebusch, and R. Fassler. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 12(6):653–663, 2000.
Rhee, S. T., and S. R. Buchman. Colocalization of c-src (pp60src) and bone morphogenetic protein 2/4 expression during mandibular distraction osteogenesis: In vivo evidence of their role within an integrin-mediated mechanotransduction pathway. Ann. Plast. Surg. 55(2):207–215, 2005.
Robling, A. G., J. Li, K. L. Shultz, W. G. Beamer, and C. H. Turner. Evidence for a skeletal mechanosensitivity gene on mouse chromosome 4. FASEB J. 17(2):324–326, 2003.
Robling, A. G., and C. H. Turner. Mechanotransduction in bone: Genetic effects on mechanosensitivity in mice. Bone 31(5):562–569, 2002.
Rossert, J., H. Eberspaecher, and B. de Crombrugghe. Separate cis-acting DNA elements of the mouse pro-alpha 1(i) collagen promoter direct expression of reporter genes to different type i collagen-producing cells in transgenic mice. J. Cell Biol. 129(5):1421–1432, 1995.
Sanchez, C., O. Gabay, C. Salvat, Y. E. Henrotin, and F. Berenbaum. Mechanical loading highly increases il-6 production and decreases opg expression by osteoblasts. Osteoarthr. Cartilage 17:473–481, 2009.
Tong, L., S. R. Buchman, M. A. Ignelzi, Jr., S. Rhee, and S. A. Goldstein. Focal adhesion kinase expression during mandibular distraction osteogenesis: Evidence for mechanotransduction. Plast. Reconstr. Surg. 111(1):211–222, 2003; discussion 23–24.
Turner, C. H., I. Owan, T. Alvey, J. Hulman, and J. M. Hock. Recruitment and proliferative responses of osteoblasts after mechanical loading in vivo determined using sustained-release bromodeoxyuridine. Bone 22(5):463–469, 1998.
Wang, Y., L. M. McNamara, M. B. Schaffler, and S. Weinbaum. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc. Natl. Acad. Sci. USA 104(40):15941–15946, 2007.
Weinstein, R. S., R. L. Jilka, A. M. Parfitt, and S. C. Manolagas. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 102(2):274–282, 1998.
Zimmerman, D., F. Jin, P. Leboy, S. Hardy, and C. Damsky. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 220(1):2–15, 2000.
Acknowledgments
The authors thank Dr. Ruth Globus, Dr. Jonathan Phillips, and Rose Mojarrab at NASA Ames Research Center, and Sara Temiyasathit at Stanford University for significant technical assistance and intellectual contributions. Funding sources included NIH Grant AR45989; NIH Grant AR54156; the US Department of Veterans Affairs; a National Science Foundation Graduate Research Fellowship; and a Veterans Affairs Pre-Doctoral Associated Health Rehabilitation Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Litzenberger, J.B., Tang, W.J., Castillo, A.B. et al. Deletion of β1 Integrins from Cortical Osteocytes Reduces Load-Induced Bone Formation. Cel. Mol. Bioeng. 2, 416–424 (2009). https://doi.org/10.1007/s12195-009-0068-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-009-0068-4