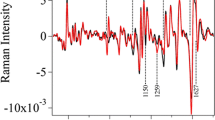
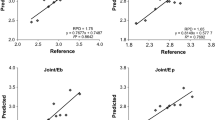
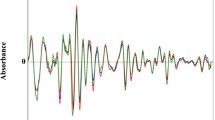
Abstract
Switchgrass (Panicum virgatum L.) is a candidate feedstock in bioenergy, and plant breeding and molecular genetic strategies are being used to improve germplasm. In order to assess these subsequent modifications, baseline biomass compositional data are needed in a relevant variety of environments. In this study, switchgrass cv. Alamo was grown in the field, greenhouse, and growth chamber and harvested into individual leaf and stem tissue components. These components were analyzed with pyrolysis vapor analysis using molecular beam mass spectrometry, Fourier transform infrared, and standard wet chemistry methods to characterize and compare the composition among the different growth environments. The details of lignin content, S/G ratios, and degree of cross-linked lignin are discussed. Multivariate approaches such as projection to latent structures regression found a very strong correlation between the lignin content obtained by standard wet chemistry methods and the two high throughput techniques employed to rapidly assess lignin in potential switchgrass candidates. The models were tested on unknown samples and verified by wet chemistry. The similar lignin content found by the two methods shows that both approaches are capable of determining lignin content in biomass in a matter of minutes.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- FTIR:
-
Fourier transform infrared spectroscopy
- L1, L2, etc.:
-
Leaf tissue 1, Leaf tissue 2, etc.
- PLS:
-
Projection to latent structures
- PyMBMS:
-
Pyrolysis vapor analysis using molecular beam mass spectrometry
- RMSEC:
-
Root mean square error of calibration
- RMSEP:
-
Root mean square error of prediction
- S1, S2, etc.:
-
Stem tissue 1, Stem tissue 2, etc.
- SD:
-
Standard deviation
References
Bransby DI, Ward CY, Rose PA, Sladden SE, Kee DD (1989) Biomass production from selected herbaceous species in the Southeastern USA. Biomass 20(1–2):187–197
McLaughlin SB, Ugarte DGDL, Garten CT, Lynd LR, Sanderson MA, Tolbert VR et al (2002) High-value renewable energy from prairie grasses. Environ Sci Technol 36(10):2122–2129
Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. PNAS 105(2):464–469
Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R et al (2008) How biotech can transform biofuels. Nat Biotechnol 26(2):169–172
Chen L, Auh C, Chen F, Cheng X, Aljoe H, Dixon RA et al (2002) Lignin deposition and associated changes in anatomy, enzyme activity, gene expression, and ruminal degradability in stems of tall fescue at different developmental stages. J Agric Food Chem 50(20):5558–5565
Dixon RA, Chen F, Guo D, Parvathi K (2001) The biosynthesis of monolignols: a “metabolic grid”, or independent pathways to guaiacyl and syringyl units? Phytochemistry 57(7):1069–1084
Vogel KP, Pedersen JF, Masterson SD, Toy JJ (1999) Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop Sci 39(1):276–279
Yuan JS, Tiller KH, Al-Ahmad H, Stewart NR, Stewart CN Jr (2008) Plants to power: bioenergy to fuel the future. Trends Plant Sci 13(8):421–429
Sticklen MB (2008) Plant genetic engineering for biofuel production: towards affordable cellulosic ethanol. Nat Rev Genet 9(6):433–443
Alexandrova KS, Denchev PD, Conger BV (1996) Micropropagation of switchgrass by node culture. Crop Sci 36(6):1709–1711
Denchev PD, Conger BV (1994) Plant-regeneration from callus-cultures of switchgrass. Crop Sci 34(6):1623–1627
Richards HA, Rudas VA, Sun H, McDaniel JK, Tomaszewski Z, Conger BV (2001) Construction of a GFP-BAR plasmid and its use for switchgrass transformation. Plant Cell Rep 20(1):48–54
Somleva MN, Tomaszewski Z, Conger BV (2002) Agrobacterium-mediated genetic transformation of switchgrass. Crop Sci 42(6):2080–2087
Tobias CM, Twigg P, Hayden DM, Vogel KP, Mitchell RM, Lazo GR et al (2005) Analysis of expressed sequence tags and the identification of associated short tandem repeats in switchgrass. Theor Appl Genet 111(5):956–964
Alexandrova KS, Denchev PD, Conger BV (1996) In vitro development of inflorescences from switchgrass nodal segments. Crop Sci 36(1):175–178
Denchev PD, Conger BV (1995) In-vitro culture of switchgrass—influence of 2, 4-D and picloram in combination with benzyladenine on callus initiation and regeneration. Plant Cell Tiss Org 40(1):43–48
Sladden SE, Bransby DI, Aiken GE (1991) Biomass yield, composition and production costs for 8 switchgrass varieties in Alabama. Biomass Bioenerg 1(2):119–122
Adler PR, Sanderson MA, Boateng AA, Weimer PI, Jung HJG (2006) Biomass yield and biofuel quality of switchgrass harvested in fall or spring. Agron J 98(6):1518–1525
Dien BS, Jung HJG, Vogel KP, Casler MD, Lamb JFS, Iten L et al (2006) Chemical composition and response to dilute-acid pretreatment and enzymatic saccharification of alfalfa, reed canarygrass, and switchgrass. Biomass Bioenerg 30(10):880–891
Grabber JH, Jung GA, Hill RR (1991) Chemical-composition of parenchyma and sclerenchyma cell-walls isolated from orchardgrass and switchgrass. Crop Sci 31(4):1058–1065
Law KN, Kokta BV, Mao CB (2001) Fibre morphology and soda-sulphite pulping of switchgrass. Bioresource Technol 77(1):1–7
El-Nashaar HM, Banowetz GM, Griffith SM, Casler MD, Vogel KP (2009) Genotypic variability in mineral composition of switchgrass. Bioresource Technol 100(5):1809–1814
Sarath G, Mitchell RB, Sattler SE, Funnell D, Pedersen JF, Graybosch RA et al (2008) Opportunities and roadblocks in utilizing forages and small grains for liquid fuels. J Ind Microbiol Biotechnol 35(5):343–354
Sarath G, Baird LM, Vogel KP, Mitchell RB (2007) Internode structure and cell wall composition in maturing tillers of switchgrass (Panicum virgatum. L). Bioresource Technol 98(16):2985–2992
Meuzelaar HLC, Haverkamp J, Hileman FD (1982) Pyrolysis mass spectrometry of recent and fossil biomaterials. Elsevier, New York
Goodacre R, Timmins EM, Burton R, Kaderbhai N, Woodward AM, Kell DB et al (1998) Rapid identification of urinary tract infection bacteria using hyperspectral whole-organism fingerprinting and artificial neural networks. Microbiol-UK 144:1157–1170
Smedsgaard J, Frisvad JC (1996) Using direct electrospray mass spectrometry in taxonomy and secondary metabolite profiling of crude fungal extracts. J Microbiol Meth 25(1):5–17
van Baar BLM (2000) Characterization of bacteria by matrix-assisted laser desorption/ionisation and electrospray mass spectrometry. FEMS Microbiol Rev 24(2):193–219
del Rio JC, Gutiérrez A, Romero J, Martínez MJ, Martínez AT (2001) Identification of residual lignin markers in eucalypt kraft pulps by Py-GC/MS. J Anal Appl Pyrol 58–59:425–439
Faix O, Bremer J, Schmidt O, Stevanovic T (1991) Monitoring of chemical changes in white-rot degraded beech wood by pyrolysis–gas chromatography and Fourier-transform infrared spectroscopy. J Anal Appl Pyrol 21:147–162
Faix O, Meier D, Grobe I (1987) Studies on isolated lignins and lignins in woody materials by pyrolysis–gas chromatography–mass spectrometry and off-line pyrolysis–gas chromatography with flame ionization detection. J Anal Appl Pyrol 11:403–416
Izumi A, Kuroda K, Ohi H, Yamaguchi A (1995) Structural analysis of lignin by pyrolysis–gas chromatography. III. Comparative studies of pyrolysis–gas chromatography and nitrobenzene oxidation for the determination method of lignin comosition in hardwood. Jpn Tappi 49(9):1339–1346
Martin F, Saiz-Jimenez C, Gonzalez-Vila FJ (1979) Pyrolysis–gas chromatography–mass spectrometry of lignins. Holzforschung 33(6):210–212
Rodrigues J, Graca J, Pereira H (2001) Influence of tree eccentric growth on syringyl/guaiacyl ratio in Eucalyptus globulus wood lignin assessed by analytical pyrolysis. J Anal Appl Pyrol 58–59:481–489
Rodrigues J, Meier D, Faix O, Pereira H (1999) Determination of tree-to-tree variation in syringyl/guaiacyl ratio of Eucalyptus globulus wood lignin by analytical pyrolysis. J Anal Appl Pyrol 48(2):121–128
Sonoda T, Ona T, Yokoi H, Ishida Y, Ohtani H, Tsuge S (2001) Quantitative analysis of detailed lignin monomer composition by pyrolysis-gas chromatography combined with preliminary acetylation of the samples. Anal Chem 73(22):5429–5435
Agblevor FA, Evans RJ, Johnson KD (1994) Molecular-beam mass-spectrometric analysis of lignocellulosic materials. 1. Herbaceous biomass. J Anal Appl Pyrol 30(2):125–144
Davis MF, Megraw R, Sewell M, Evans R, Neale D, West D, et al (1999) Application of pyrolysis molecular beam mass spectrometry for the determination of loblolly pine and hybrid poplar cell wall composition. Paper presented at the TAPPI Pulping Conference, Orlando, FL, 31 October–4 November 1999 3: 1077–1081
Evans RJ, Milne TA (1987) Molecular characterization of the pyrolysis of biomass. 2. Applications. Energ Fuel 1(4):311–319
Evans RJ, Milne TA, Soltys MN (1986) Direct mass-spectrometric studies of the pyrolysis of carbonaceous fuels. III. Primary pyrolysis of lignin. J Anal Appl Pyrol 9(3):207–236
Kelley SS, Jellison J, Goodell B (2002) Use of NIR and pyrolysis-MBMS coupled with multivariate analysis for detecting the chemical changes associated with brown-rot biodegradation of spruce wood. FEMS Microbiol Lett 209(1):107–111
Davis MF, Tuskan GA, Payne P, Tschaplinski TJ, Meilan R (2006) Assessment of Populus wood chemistry following the introduction of a Bt toxin gene. Tree Physiol 26(5):557–564
Sykes R, Kodrzycki B, Tuskan GA, Foutz K, Davis M (2008) Within tree variability of lignin composition in Populus. Wood Sci Technol 42(8):649–661
Labbé N, Rials TG, Kelley SS, Cheng ZM, Kim JY, Li Y (2005) FT-IR imaging and pyrolysis–molecular beam mass spectrometry: new tools to investigate wood tissues. Wood Sci Technol 39(1):61–76
Chen L, Carpita NC, Reiter WD, Wilson RH, Jeffries C, McCann MC (1998) A rapid method to screen for cell-wall mutants using discriminant analysis of Fourier transform infrared spectra. Plant J 16(3):385–92
Kataoka Y, Kondo T (1999) Quantitative analysis for the cellulose I alpha crystalline phase in developing wood cell walls. Int J Biol Macromol 24(1):37–41
Moore KJ, Moser LE, Vogel KP, Waller SS, Johnson BE, Pedersen JF (1991) Describing and quantifying growth stages of perennial forage grasses. Agron J 83(6):1073–1077
Evans RJ, Milne TA (1987) Molecular characterization of the pyrolysis of biomass. Energ Fuel 1(2):123–137
Tuskan G, West D, Bradshaw HD, Neale D, Sewell M, Wheeler N et al (1999) Two high-throughput techniques for determining wood properties as part of a molecular genetics analysis of hybrid poplar and loblolly pine. Appl Biochem Biotechnol 77(1–3):55–65
Shin EJ, Hajaligol MR, Rasouli F (2003) Characterizing biomatrix materials using pyrolysis molecular beam mass spectrometer and pattern. J Anal Appl Pyrol 68–69:213–229
Stewart D, Yahiaoui N, McDougall GJ, Myton K, Marque C, Boudet AM et al (1997) Fourier-transform infrared and Raman spectroscopic evidence for the incorporation of cinnamaldehydes into the lignin of transgenic tobacco (Nicotiana tabacum L.) plants with reduced expression of cinnamyl alcohol dehydrogenase. Planta 201(3):311–318
Zhong R, Morrison WH III, Himmelsbach DS, Poole FL II, Ye Z-H (2000) Essential role of caffeoyl coenzyme A O-methyltransferase in lignin biosynthesis in woody poplar plants. Plant Physiol 124(2):563–577
Jung HJG, Vogel KP (1992) Lignification of switchgrass (Panicum virgatum) and big bluestem (Andropogon gerardii) plant-parts during maturation and its effect on fiber degradability. J Sci Food Agr 59(2):169–176
Okuyama T, Takeda H, Yamamoto H (1998) Relation between growth stress and lignin concentration in the cell wall: ultraviolet microscopic spectral analysis. J Wood Sci 44(2):83–89
Yeh T-F, Goldfarb B, Chang H-M, Peszlen I, Braun JL, Kadla JF (2005) Comparison of morphological and chemical properties between juvenile wood and compression wood of loblolly pine. Holzforschung 59(6):669–674
Acknowledgments
The authors would like to acknowledge Mitra Mazarei and Murali Raghavendra for input on the manuscript. This work was funded by the Southeastern Sun Grant Initiative grant number DOT 0T0S5907G00050 and the Bioenergy Science Center (BESC). BESC is a US Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mann, D.G.J., Labbé, N., Sykes, R.W. et al. Rapid Assessment of Lignin Content and Structure in Switchgrass (Panicum virgatum L.) Grown Under Different Environmental Conditions. Bioenerg. Res. 2, 246–256 (2009). https://doi.org/10.1007/s12155-009-9054-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-009-9054-x