Abstract
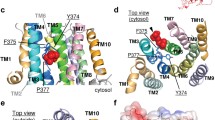
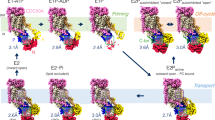
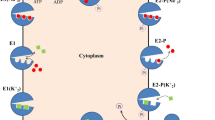
The P4 subfamily of P-type ATPases includes phospholipid transporters. Moving such bulky amphipathic substrate molecules across the membrane poses unique mechanistic problems. Recently, three papers from three different laboratories have offered insights into some of these problems. One effect of these experiments will be to ignite a healthy debate about the path through the enzyme taken by the substrate. A second effect is to suggest a counterintuitive model for the critical substrate-binding site. By putting concrete hypotheses into play, these papers finally provide a foundation for investigations of mechanism for these proteins.


Similar content being viewed by others
References
Albers RW (1967) Biochemical aspects of active transport. Annu Rev Biochem 36:727–756. doi:10.1146/annurev.bi.36.070167.003455
Axelsen KB, Palmgren MG (1998) Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol 46(1):84–101
Baldridge RD, Graham TR (2012) Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc Natl Acad Sci U S A 109(6):E290–E298. doi:10.1073/pnas.1115725109[doi]
Bryde S, Hennrich H, Verhulst PM, Devaux PF, Lenoir G, Holthuis JC (2010) CDC50 proteins are critical components of the human class-1 P4-ATPase transport machinery. J Biol Chem 285(52):40562–40572
Coleman JA, Molday RS (2011) Critical role of the {beta}-subunit CDC50A in the stable expression, assembly, subcellular localization and lipid transport activity of the P4-ATPase ATP8A2. J Biol Chem 286:17205–17216
Coleman JA, Vestergaard AL, Molday RS, Vilsen B, Peter Andersen J (2012) Critical role of a transmembrane lysine in aminophospholipid transport by mammalian photoreceptor P4-ATPase ATP8A2. Proc Natl Acad Sci U S A 109(5):1449–1454. doi:10.1073/pnas.1108862109[doi]
Ding J, Wu Z, Crider BP, Ma Y, Li X, Slaughter C, Gong L, Xie XS (2000) Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem 275(30):23378–23386
Hall MP, Huestis WH (1994) Phosphatidylserine headgroup diastereomers translocate equivalently across human erythrocyte membranes. Biochim Biophys Acta Biomembr 1190(2):243–247
Halleck MS, Pradhan D, Blackman C, Berkes C, Williamson P, Schlegel RA (1998) Multiple members of a third subfamily of P-type ATPases identified by genomic sequences and ESTs. Genome Res 8(4):354–361
Jacquot A, Montigny C, Hennrich H, Barry R, le Maire M, Jaxel C, Holthuis J, Champeil P, Lenoir G (2012) Phosphatidylserine stimulation of Drs2p.Cdc50p lipid translocase dephosphorylation is controlled by phosphatidylinositol-4-phosphate. J Biol Chem 287(16):13249–13261. doi:10.1074/jbc.M111.313916
Jardetzky O (1966) Simple allosteric model for membrane pumps. Nature 211(5052):969–970
Lenoir G, Williamson P, Holthuis JC (2007) On the origin of lipid asymmetry: the flip side of ion transport. Curr Opin Chem Biol 11:1–8
Lenoir G, Williamson P, Puts CF, Holthuis JC (2009) Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter drs2p. J Biol Chem 284(27):17956–17967
Obara K, Miyashita N, Xu C, Toyoshima I, Sugita Y, Inesi G, Toyoshima C (2005) Structural role of countertransport revealed in Ca(2+) pump crystal structure in the absence of Ca(2+). Proc Natl Acad Sci U S A 102(41):14489–14496
Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P (2007) The structural basis of calcium transport by the calcium pump. Nature 450(7172):1036–1042
Olesen C, Sorensen TL, Nielsen RC, Moller JV, Nissen P (2004) Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306(5705):2251–2255
Palmgren MG, Nissen P (2011) P-type ATPases. Annu Rev Biophys 40:243–266
Pedersen BP, Buch-Pedersen MJ, Morth JP, Palmgren MG, Nissen P (2007) Crystal structure of the plasma membrane proton pump. Nature 450(7172):1111–1114
Pomorski T, Holthuis JC, Herrmann A, van Meer G (2004) Tracking down lipid flippases and their biological functions. J Cell Sci 117(Pt 6):805–813
Pomorski T, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JC (2003) Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell 14(3):1240–1254
Post RL, Hegyvary C, Kume S (1972) Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem 247(20):6530–6540
Post RL, Kume S, Tobin T, Orcutt B, Sen AK (1969) Flexibility of an active center in sodium-plus-potassium adenosine triphosphatase. J Gen Physiol 54(1):306–326
Ripmaster TL, Vaughn GP, Woolford JL Jr (1993) DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol Cell Biol 13(12):7901–7912
Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K (2004) Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell 15(7):3418–3432
Takatsu H, Baba K, Shima T, Umino H, Kato U, Umeda M, Nakayama K, Shin HW (2011) ATP9B, a P4-ATPase (a putative aminophospholipid translocase), localizes to the trans-Golgi network in a CDC50-independent manner. J Biol Chem 286:38159–38167
Tang X, Halleck MS, Schlegel RA, Williamson P (1996) A subfamily of P-type ATPases with aminophospholipid transporting activity. Science 272(5267):1495–1497
Toyoshima C, Nomura H, Tsuda T (2004) Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432(7015):361–368
Zachowski A, Henry JP, Devaux PF (1989) Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature 340(6228):75–76
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stone, A., Williamson, P. Outside of the box: recent news about phospholipid translocation by P4 ATPases. J Chem Biol 5, 131–136 (2012). https://doi.org/10.1007/s12154-012-0078-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12154-012-0078-x