Abstract
Theoretical models and empirical analyses have combined to suggest that, for some ranges of climate, alternative stable states in vegetation structure may be possible: positive feedbacks with fire may differentiate savanna from forest, while positive feedbacks with hydrological processes may differentiate grassland from savanna. However, existing models have largely failed to include spatially explicit fire spread, dispersal, and hydrological processes, and as such ignore key features of vegetation systems. In this paper, we incorporate spatial and stochastic effects to form a comprehensive and realistic description of the dynamics of vegetation growth. By using novel computational tools such as path-sampling, we investigate the effect of noise and spatial coupling on the stability and the dynamics of forest-savanna transitions. Spatial effects change model behaviors: We find that spatial interactions between savanna and grassland result in the eventual nucleation and propagation of one of the states to invade the other. Which state wins depends on parameter values, which in turn depend on environmental conditions. Meanwhile, transitions between savanna and forest occur much less frequently, because an event such as a period of fire suppression in savanna or a fire in forest must be larger to successfully invade a landscape; this results in transitions between savanna and forest that, although not strictly history dependent, look temporally similar to hysteresis because the time lags involved in transitions are so large.















Similar content being viewed by others
Notes
Note that technically, the value of Tgrassland varies with w (see Appendix A for asymptotic expressions of Tgrassland, Tsavanna, and Tforest). Here and subsequently, we omit writing out the explicit dependence on w for simplicity of notation.
Note that since we consider a rainfall gradient in the i direction, we shall denote by \(\mathcal {T}_{i,n}\) the average coverage in the j direction at patch i.
References
Accatino F, De Michele C, Vezzoli R, Donzelli D, Scholes RJ (2010) Tree–grass co-existence in savanna: interactions of rain and fire. J Theor Biol 267(2):235–242
Aleman JC, Staver AC (2018) Spatial patterns in the global distributions of savanna and forest. Global Ecology and Biogeography
Aleman JC, Blarquez O, Staver CA (2016) Land-use change outweighs projected effects of changing rainfall on tree cover in Sub-Saharan Africa. Glob Chang Biol 22(9):3013–3025
Archibald S, Roy DP, Wilgen V, Brian W, Scholes RJ (2009) What limits fire? An examination of drivers of burnt area in Southern Africa. Glob Chang Biol 15(3):613–630
Archibald S, Staver AC, Levin SA (2012) Evolution of human-driven fire regimes in Africa. Proc Natl Acad Sci 109(3):847–852
Baudena M, Rietkerk M (2013) Complexity and coexistence in a simple spatial model for arid savanna ecosystems. Theor Ecol 6(2):131–141
Beckage B, Ellingwood C (2008) Fire feedbacks with vegetation and alternative stable states. Complex Syst 18(1):159
Beckage B, Platt WJ, Gross LJ (2009) Vegetation, fire, and feedbacks: a disturbance-mediated model of savannas. Am Nat 174(6):805–818
Belsky AJ (1990) Tree/grass ratios in East African savannas: a comparison of existing models, J Biogeogr, 483–489
Belsky AJ, Canham CD (1994) Forest gaps and isolated savanna trees. BioScience 44(2):77–84
Belsky A, Mwonga S, Amundson R, Duxbury J, Ali A (1993) Comparative effects of isolated trees on their undercanopy environments in high-and low-rainfall savannas. J Appl Ecol, 143–155
Binder K (1987) Theory of first-order phase transitions. Rep Progress Phys 50(7):783
Bolhuis PG, Chandler D, Dellago C, Geissler PL (2002) Transition path sampling: throwing ropes over rough mountain passes, in the dark. Ann Rev Phys Chem 53(1):291–318
Bonachela JA, Pringle RM, Sheffer E, Coverdale TC, Guyton JA, Caylor KK, Levin SA, Tarnita CE (2015) Termite mounds can increase the robustness of dryland ecosystems to climatic change. Science 347(6222):651–655
Bond WJ (2008) What limits trees in C4 grasslands and savannas? Ann Rev Ecol Evol Systemat 39:641–659
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. N Phytol 165(2):525–538
Brookman-Amissah J, Hall J, Swaine M, Attakorah J (1980) A re-assessment of a fire protection experiment in north-eastern Ghana savanna, J Appl Ecol, 85–99
Brown JR, Archer S (1999) Shrub invasion of grassland: recruitment is continuous and not regulated by herbaceous biomass or density. Ecology 80(7):2385–2396
Bucini G, Hanan NP (2007) A continental-scale analysis of tree cover in African savannas. Glob Ecol Biogeogr 16(5):593–605
Bunney K, Bond WJ, Henley M (2017) Seed dispersal kernel of the largest surviving megaherbivore—the African savanna elephant. Biotropica 49(3):395–401
Caylor KK, Scanlon TM, Rodriguez-Iturbe I (2009) Ecohydrological optimization of pattern and processes in water-limited ecosystems: a trade-off-based hypothesis. Water Resour Res 45:8
Chandler D (1987) Introduction to modern statistical mechanics. Introduction to modern statistical mechanics, by David Chandler, pp 288 Foreword by David Chandler Oxford University Press. Sep 1987 ISBN-10: 0195042778 ISBN-13; 9780195042771, p 288
Charles-Dominique T, Staver AC, Midgley G, Bond W (2015) Functional differentiation of biomes in an African savanna/forest mosaic. S Afr J Bot 101:82–90
Colgan M, Asner G, Levick SR, Martin R, Chadwick O (2012) Topo-edaphic controls over woody plant biomass in South African savannas. Biogeosciences 9:1809–1821
Dantas V d L, Hirota M, Oliveira RS, Pausas JG (2016) Disturbance maintains alternative biome states. Ecol Lett 19(1):12–19
Dellago C, Bolhuis PG, Csajka FS, Chandler D (1998) Transition path sampling and the calculation of rate constants. J Chem Phys 108:1964. https://doi.org/10.1063/1.475562. http://link.aip.org/link/?JCPSA6/108/1964/1
Dohn J, Dembélé F, Karembé M, Moustakas A, Amévor K A, Hanan NP (2013) Tree effects on grass growth in savannas: competition, facilitation and the stress-gradient hypothesis. J Ecol 101(1):202–209
Donat MG, Alexander LV, Yang H, Durre I, Vose R, Caesar J (2013) Global land-based datasets for monitoring climatic extremes. Bull Am Meteorol Soc 94(7):997–1006
Durrett R, Zhang Y (2015) Coexistence of grass, saplings and trees in the Staver–Levin forest model. Ann Appl Probab 25(6):3434–3464
Favier C, Chave J, Fabing A, Schwartz D, Dubois MA (2004a) Modelling forest–savanna mosaic dynamics in man-influenced environments: effects of fire, climate and soil heterogeneity. Ecol Model 171(1):85–102
Favier C, De Namur C, Dubois MA (2004b) Forest progression modes in littoral Congo, Central Atlantic Africa. J Biogeogr 31(9):1445–1461
February EC, Higgins SI, Bond WJ, Swemmer L (2013) Influence of competition and rainfall manipulation on the growth responses of savanna trees and grasses. Ecology 94(5):1155–1164
Franz TE, King EG, Caylor KK, Robinson DA (2011) Coupling vegetation organization patterns to soil resource heterogeneity in a Central Kenyan dryland using geophysical imagery. Water Resour Res 47:7
Franz TE, Caylor KK, King EG, Nordbotten JM, Celia MA, Rodríguez-Iturbe I (2012) An ecohydrological approach to predicting hillslope-scale vegetation patterns in dryland ecosystems. Water Resour Res 48:1
Freidlin MI, Wentzell AD (2012) Random perturbations of dynamical systems, vol 260. Springer Science & Business Media
García-Ojalvo J, Sancho J (2012) Noise in spatially extended systems. Springer Science & Business Media
Gillespie DT (2000) The chemical Langevin equation. J Chem Phys 113(1):297–306
Goetze D, Hörsch B, Porembski S (2006) Dynamics of forest–savanna mosaics in north-eastern ivory coast from 1954 to 2002. J Biogeogr 33(4):653–664
Govender N, Trollope WS, Van Wilgen BW (2006) The effect of fire season, fire frequency, rainfall and management on fire intensity in savanna vegetation in South Africa. J Appl Ecol 43(4):748–758
Gray EF, Bond WJ (2015) Soil nutrients in an African forest/savanna mosaic: drivers or driven? S Afr J Bot 101:66–72
Hanan NP, Tredennick AT, Prihodko L, Bucini G, Dohn J (2014) Analysis of stable states in global savannas: is the cart pulling the horse? Glob Ecol Biogeogr 23(3):259–263
Hansen MC, Potapov PV, Moore R, Hancher M, Turubanova S, Tyukavina A, Thau D, Stehman S, Goetz S, Loveland T et al (2013) High-resolution global maps of 21st-century forest cover change. Science 342(6160):850–853
Hantson S, Lasslop G, Kloster S, Chuvieco E (2015) Anthropogenic effects on global mean fire size. Int J Wildland Fire 24(5):589–596
Hantson S, Scheffer M, Pueyo S, Xu C, Lasslop G, Nes EH, Holmgren M, Mendelsohn J (2017) Rare, intense, big fires dominate the global tropics under drier conditions. Sci Rep 7(1):14374
Hennenberg KJ, Fischer F, Kouadio K, Goetze D, Orthmann B, Linsenmair KE, Jeltsch F, Porembski S (2006) Phytomass and fire occurrence along forest-savanna transects in the Comoe National Park, Ivory Coast, J Trop Ecol, 303–311
Higgins SI, Scheiter S (2012) Atmospheric CO2 forces abrupt vegetation shifts locally, but not globally. Nature 488(7410):209–212
Higgins SI, Bond WJ, Trollope WS (2000) Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J Ecol 88(2):213–229
Higgins SI, Bond WJ, February EC, Bronn A, Euston-Brown DI, Enslin B, Govender N, Rademan L, O’Regan S, Potgieter AL et al (2007) Effects of four decades of fire manipulation on woody vegetation structure in savanna. Ecology 88(5):1119–1125
Hirota M, Holmgren M, Van Nes EH, Scheffer M (2011) Global resilience of tropical forest and savanna to critical transitions. Science 334(6053):232–235
Hoffmann WA, Orthen B, Do Nascimento PKV (2003) Comparative fire ecology of tropical savanna and forest trees. Funct Ecol 17(6):720–726
Hoffmann WA, Adasme R, Haridasan M, T de Carvalho M, Geiger EL, Pereira MA, Gotsch SG, Franco AC (2009) Tree topkill, not mortality, governs the dynamics of savanna–forest boundaries under frequent fire in Central Brazil. Ecology 90(5):1326–1337
Holdo RM (2013) Revisiting the two-layer hypothesis: coexistence of alternative functional rooting strategies in savannas. PLoS One 8(8):e69625
Holdridge LR (1947) Determination of world plant formations from simple climatic data. Science 105 (2727):367–368
Khasminskii R (2011) Stochastic stability of differential equations, vol 66. Springer Science & Business Media
King EG, Franz TE, Caylor KK (2012) Ecohydrological interactions in a degraded two-phase mosaic dryland: implications for regime shifts, resilience, and restoration. Ecohydrology 5(6):733–745
Landau L, Lifshitz E (1980) Statistical physics, part I. Pergamon. Oxford
Lefever R, Lejeune O (1997) On the origin of tiger bush. Bull Math Biol 59(2):263–294
Levin S (1979) Non-uniform stable solutions to reaction-diffusion equations: applications to ecological pattern formation. In: Pattern formation by dynamic systems and pattern recognition. Springer, pp 210–222
Levin SA, Muller-Landau HC (2000) The evolution of dispersal and seed size in plant communities. Evol Ecol Res 2(4):409–435
Levin SA, Muller-Landau HC, Nathan R, Chave J (2003) The ecology and evolution of seed dispersal: a theoretical perspective. Ann Rev Ecol Evol Syst 34(1):575–604
Li Q, Weinan E (2015) The free action of nonequilibrium dynamics. J Stat Phys 161(2):300–325
Lloyd J, Bird MI, Vellen L, Miranda AC, Veenendaal EM, Djagbletey G, Miranda HS, Cook G, Farquhar GD (2008) Contributions of woody and herbaceous vegetation to tropical savanna ecosystem productivity: a quasi-global estimate. Tree Physiol 28(3):451–468
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E (1953) Equation of state calculations by fast computing machines. J Chem Phys 21(6):1087–1092. https://doi.org/10.1063/1.1699114. http://jcp.aip.org/resource/1/jcpsa6/v21/i6/p1087_s1?bypassSSO=1
Moreira AG (2000) Effects of fire protection on savanna structure in Central Brazil. J Biogeogr 27(4):1021–1029
Moustakas A, Kunin WE, Cameron TC, Sankaran M (2013) Facilitation or competition? Tree effects on grass biomass across a precipitation gradient. PLoS One 8(2):e57025
Nicholson SE, Some B, McCollum J, Nelkin E, Klotter D, Berte Y, Diallo B, Gaye I, Kpabeba G, Ndiaye O et al (2003) Validation of TRMM and other rainfall estimates with a high-density gauge dataset for West Africa. Part I: validation of GPCC rainfall product and pre-TRMM satellite and blended products. J Appl Meteorol 42(10):1337–1354
Nishimori H (2001) Statistical physics of spin glasses and information processing: an introduction. Clarendon Press, p 111
Ogata Y (1989) A Monte Carlo method for high dimensional integration. Numer Math 55(2):137–157
Pellegrini AF, Hoffmann WA, Franco AC (2014) Carbon accumulation and nitrogen pool recovery during transitions from savanna to forest in Central Brazil. Ecology 95(2):342–352
Risken H (1984) Fokker-Planck equation. In: The Fokker-Planck equation. Springer, pp 63–95
Rodriguez-Iturbe I (2000) Ecohydrology: a hydrologic perspective of climate-soil-vegetation dynamics. Water Resour Res 36(1):3–9
Sankaran M, Hanan NP, Scholes RJ, Ratnam J, et al. (2005) Determinants of woody cover in African savannas. Nature 438(7069):846
Sankaran M, Ratnam J, Hanan N (2008) Woody cover in African savannas: the role of resources, fire and herbivory. Glob Ecol Biogeogr 17(2):236–245
Scanlon TM, Caylor KK, Levin SA, Rodriguez-Iturbe I (2007) Positive feedbacks promote power-law clustering of Kalahari vegetation. Nature 449(7159):209–212
Schertzer E, Staver AC (2018) Fire spread and the issue of community-level selection in the evolution of flammability. J R Soc Interf 15(147):20180444
Schertzer E, Staver AC, Levin SA (2015) Implications of the spatial dynamics of fire spread for the bistability of savanna and forest. J Math Biol 70(1–2):329–341
Scholes R, Archer S (1997) Tree-grass interactions in savannas. Ann Rev Ecol Syst 28(1):517–544
Schutz AEN, Bond WJ, Cramer MD (2009) Juggling carbon: allocation patterns of a dominant tree in a fire-prone savanna. Oecologia 160(2):235
Scogings PF (2014) Large herbivores and season independently affect woody stem circumference increment in a semi-arid African savanna. Plant Ecol 215(12):1433–1443
Silva LC, Sternberg L, Haridasan M, Hoffmann WA, Miralles-Wilhelm F, Franco AC (2008) Expansion of gallery forests into Central Brazilian savannas. Glob Change Biol 14(9):2108– 2118
Smit IP, Smit CF, Govender N, Mvd Linde, MacFadyen S (2013) Rainfall, geology and landscape position generate large-scale spatiotemporal fire pattern heterogeneity in an African savanna. Ecography 36(4):447–459
Staal A, Dekker SC, Xu C, van Nes EH (2016) Bistability, spatial interaction, and the distribution of tropical forests and savannas. Ecosystems 19(6):1080–1091
Staver AC (2017) Prediction and scale in savanna ecosystems. New Phytologist
Staver AC, Hansen MC (2015) Analysis of stable states in global savannas: is the cart pulling the horse?–a comment. Glob Ecol Biogeogr 24(8):985–987
Staver AC, Levin SA (2012) Integrating theoretical climate and fire effects on savanna and forest systems. Am Nat 180(2):211–224
Staver AC, Archibald S, Levin SA (2011a) The global extent and determinants of savanna and forest as alternative biome states. Science 334(6053):230–232
Staver AC, Archibald S, Levin SA (2011b) Tree cover in sub-saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92(5):1063–1072
Staver AC, Beckett H, Graf J (2017a) Ch 3: long-term vegetation dynamics within the Hluhluwe-iMfolozi Park. Conserving Africa’s mega-diversity in the anthropocene: the Hluhluwe-iMfolozi Park Story, 56
Staver AC, Botha J, Hedin L (2017b) Soils and fire jointly determine vegetation structure in an African savanna. N Phytol 216(4):1151–1160
Tarnita CE, Bonachela JA, Sheffer E, Guyton JA, Coverdale TC, Long RA, Pringle RM (2017) A theoretical foundation for multi-scale regular vegetation patterns. Nature 541(7637):398–401
Trollope W, Tainton N (1986) Effect of fire intensity on the grass and bush components of the Eastern Cape Thornveld. J Grassland Soc Southern Africa 3(2):37–42
van de Leemput IA, van Nes EH, Scheffer M (2015) Resilience of alternative states in spatially extended ecosystems. PloS one 10(2):e0116859
Van Wilgen B, Govender N, Biggs H, Ntsala D, Funda X (2004) Response of savanna fire regimes to changing fire-management policies in a large African national park. Conserv Biol 18(6):1533–1540
Vanden-Eijnden E, Westdickenberg MG (2008) Rare events in stochastic partial differential equations on large spatial domains. J Stat Phys 131(6):1023–1038
Veenendaal EM, Torello-Raventos M, Miranda HS, Sato NM, Oliveras I, van Langevelde F, Asner GP, Lloyd J (2018) On the relationship between fire regime and vegetation structure in the tropics. N Phytol 218(1):153–166
Veldman JW, Buisson E, Durigan G, Fernandes GW, Le Stradic S, Mahy G, Negreiros D, Overbeck GE, Veldman RG, Zaloumis NP et al (2015) Toward an old-growth concept for grasslands, savannas, and woodlands. Front Ecol Environ 13(3):154–162
Weinan E, Zhou X, Cheng X (2012) Subcritical bifurcation in spatially extended systems. Nonlinearity 25(3):761
Westra S, Alexander LV, Zwiers FW (2013) Global increasing trends in annual maximum daily precipitation. J Climate 26(11):3904–3918
Whittaker RH (1962) Classification of natural communities. Bot Rev 28(1):1–239
Wiedemeier DB, Bloesch U, Hagedorn F (2012) Stable forest–savanna mosaic in north-western Tanzania: local-scale evidence from δ13C signatures and 14C ages of soil fractions. J Biogeograp 39(2):247–257
Zelnik YR, Meron E (2018) Regime shifts by front dynamics. Ecological Indicators
Acknowledgments
Qianxiao Li is supported by Agency for Science, Technology and Research, Singapore. A Carla Staver is supported by the National Science Foundation under grant DMS-1615531. E. Weinan is partially supported by Department of Energy DE-SC0009248 and Office of Naval Research N00014-13-1-0338. Simon Levin is pleased to acknowledge the support of the National Science Foundation under grant DMS-1615585 to Princeton University.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is dedicated in honor of Professor Alan Hastings’ 65th birthday.
Appendices
Appendix 1. Detailed stability analysis of the well-mixed model
In this section, we analyze in detail the well-mixed or mean-field model considered in this paper. To simplify the subsequent analytical computations, some results will be presented as asymptotic in δ, which we assume throughout this work to be small. Note that non-asymptotic analytical computations can always be carried out, but it gets cumbersome to write down the full expressions.
Consider the well-mixed model (μ = ν = 1)
To obtain steady states, we set \(\dot {T}=\dot {S}=0\) to get
which simplifies to
Let us assume that β is sufficiently large so that K(T) has only one asymptote, i.e., the numerator of K(T) has only one zero. This is equivalent to the condition that the discriminant of the cubic polynomial δ − T(δ + β(T − 1)T + 1) is negative, which implies
In this case, the only asymptote of K(T) is at
One can check that Tasymp < 1 and approaches 1 as β →∞. Therefore, K(T) > 0 for T ∈ [0,Tasymp) and K(T) < 0 for T ∈ [Tasymp, 1]. Consequently, wh(T) can only intersect K(T) in the range [0,Tasymp). The number of intersections depends on the behavior of K(T): if K(T) is monotone increasing in [0,Tasymp), then there is only one intersection; if K(T) has turning points in [0,Tasymp), there can be multiple intersections. See Fig. 1. To investigate turning points, we set K′(T) = 0 to yield the equation
whose solutions are
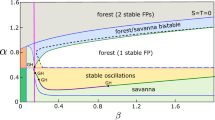
If δ > 1/8, these solutions are complex and consequently, K has no turning points for T ∈ [0, 1]. Alternatively δ < 1/8, where T1,T2 ∈ [0, 1]. In particular, T1 is a local maximum and T2 is a local minimum of K. For δ = 1/8, T1 = T2 is a point of inflection. We shall hereafter assume that δ < 1/8 so that there is a possibility, as we subsequently demonstrate, of producing up to 3 stable steady states. Lastly, we require T2 < 0.4 in order to produce two low-coverage (< 40% tree coverage) steady states, which translates to the condition
We also assume that the two conditions (22), (27) on β are satisfied.
To analyze stability of the steady states, we investigate the eigenvalues of the Jacobian matrix, which for T≠ 0.4 is
We have
Since the trace (which is equal to the sum of the eigenvalues) is negative, at least one of them must have negative real part. The sign of the real part of the other eigenvalue then determines the stability of the steady state. In particular, if the determinant (which is equal to the product of eigenvalues) is positive, then the steady state is stable. If it is negative, we then have an unstable saddle point. Eliminating S at a steady state, we have
First, we investigate the stability of Tgrassland, the steady state with the smallest T value. This exists as long as w < K(T1), and must satisfy Tgrassland < T1 and so h(Tgrassland) = 1. In particular, Tgrassland solves w = K(Tgrassland) and admits the asymptotic expression
This gives \(\text {Det}[J(T_{\text {grassland}})] = 1+w + \mathcal {O} (\delta )\), which is positive for small δ. Hence, Tgrassland is stable. Similarly, Tsavanna is the largest real solution distinct from Tgrassland of w = K(Tsavanna). This gives
which is real if \(w > w_{1} = 2(2+\sqrt {\beta })/(\beta -4) + \mathcal {O}(\delta )\). Hence,
is positive to leading order in δ. This establishes the stability of Tsavanna. Note that by a similar calculation, the solution of w = K(T) between Tgrassland and Tsavanna is given by
which one can check is a saddle point with Det[J(T)] < 0. Finally, Tforest is obtained as the largest real solution of 1.2w = K(T) that also satisfies T > 0.4. This has the asymptotic expression
and it is real and greater than 0.4 if \(w>w_{2} = 1.2(4\beta +25)/(6\beta - 25) + \mathcal {O}(\delta )\). As before, one can check that in this case, DetJ(Tforest) > 0 and so Tforest is stable. This tri-stable situation persists until w > K(0.4), i.e., \(w > w_{3} = (4\beta +25)/(6\beta -25) + \mathcal {O}(\delta )\), after which Tsavanna > 0.4 and thus is no longer an allowed steady state. Finally, Tgrassland also vanishes when w > w4 = K(T1), after which we are left with a unique stable steady state Tforest.
Appendix 2. The effect of noise and spatial coupling on multi-stability
In this section, we discuss through a concrete 1D example the effect of noise and spatial coupling on multi-stable dynamical systems. Fix an unequal double-well potential \(V(u) = \tfrac {1}{2}{(u-1)}^{2}{(u+1)}^{2} + \delta u\) where |δ|≪ 1. The potential V (u) has two local minima u+,u− close to ± 1 and the global minimum is u+ (resp. u−) if δ < 0 (resp. δ > 0). Consider the 1D dynamical system given by the gradient flow
It is clear that for any initial condition except the saddle point of V, u converges to either u+ or u−, depending on the initial condition. In other words, the system is bistable in the sense that there are two stable equilibrium points of the dynamics. One can impart a dynamical meaning to this bistability by considering the noisy version of Eq. 37,
where η is a 1D white noise and σ ≪ 1. In this case, most of the time u is close to one of u±, but it occasionally jumps between them. The rate of jumping is determined by V and in particular, one can check that the invariant (equilibrium) distribution of u is
Hence, with the small noise, the system is bistable in the true dynamical sense and one can talk about transition events between u±, which is not possible in Eq. 37 whose evolution is entirely determined by its initial condition.
The evolution of Eq. 41 with an initialized front between alternative stable states of the well-mixed model. We took r = 10,σ = 1e − 2, and n = 100. Instead of bistability, we observe propagating fronts that selects u− over u+ effectively deterministically. The bistability decays in the thermodynamic limit
The addition of spatial coupling fundamentally changes the bistable picture described above. Let us consider n copies of the dynamics (37) with a spatial coupling in the form
where periodic boundary conditions un+ 1 ≡ u0 and u− 1 ≡ un are enforced. Let us also define the macrostate \(\bar {u}\) as the spatial average \(\bar {u}:=\tfrac {1}{n} {\sum }_{i=1}^{n} u_{i}\). Evidently, if every ui = u+ (or u−) then the coupling term vanishes and one is tempted to conclude that \(\bar {u}\) behaves like the well-mixed model (38). However, this is not the case, especially in the limit n →∞.
Suppose that we initialize the systems so that all of ui(0) = u− except for a interval of values where ui(0) = u+. Although the interior of each of these clusters will fluctuate stably around either u+ or u−, the interfaces between them will move deterministically depending on the sign of δ. For example, if δ > 0 (no matter how small), the interface will move in such a way as to destroy the cluster of states with values equal to u+. This is known as “propagation.” This will happen as long as a “nucleation” event happens, i.e., there is a sufficiently large cluster of ui such that ui = u− so that a well-defined interface is formed. Once formed, the propagation effect will take over the system. The smallest size m of the nucleus required is called the critical nucleus size. It can be shown that m the transition u+ → u− is independent of n for δ > 0, whereas the critical nucleus size of the reverse transition grows linearly in system size (this must be the case since these critical nuclei are complement of each other). See for example, Vanden-Eijnden and Westdickenberg (2008) and Weinan et al. (2012) for concrete analysis of such phenomena.
Hence, spatial coupling introduces a fundamental asymmetry between macrostates \(\bar {u} = u_{+}\) and \(\bar {u} = u_{-}\) for δ≠ 0 that manifests itself in the thermodynamic limit n →∞. If δ > 0, then the transition from \(\bar {u} = u_{+}\) to \(\bar {u} = u_{-}\) requires only a cluster of m (independent of n) ui’s to jump to u−, and this happens with probability approaching 1 as n grows due to the random fluctuations. On the other hand, the reverse transition requires n − m clusters to be formed due to random fluctuations. This probability of this happening per unit time has leading exponential form κn−m (where κ is the rate per unit time of each patch jumping). This decays exponentially as n →∞. The reverse case is true for δ < 0. Hence, δ = 0 can be regarded as a well-defined first-order phase transition point of the spatial system in the thermodynamic limit, where the macrostates u± exchange relative stability.
These effects highlight the difference between phase transition in spatial systems and bistability in mean-field systems.
Appendix 3. Free action and its computation
In this section, we give a formal introduction to the concept of free action, which can be thought of as a non-equilibrium analogue of the free energy for studying transition dynamics in non-equilibrium systems. The reader is referred to Li and Weinan (2015) for a more complete exposition.
C.1 The general microscopic model
Consider a system of over-damped Langevin equations for x(t) = (x1(t),…,xn(t)) in the form
where \(x_{i}(t) \in \mathbb {R}^{d}\), \(f_{i} : \mathbb {R}^{d}\rightarrow \mathbb {R}^{d}\) and ηi(t) are d-dimensional white noises. The equation is interpreted in the Itô sense and n is the system size. We hereafter denote f = (f1,…,fn). Let us also denote by ρn the invariant distribution of x(t), which exists under mild conditions, e.g., if there exists R > 0 such that xTf(x) < − 1/R for all x such that |x| > R (Khasminskii 2011). The invariant distribution ρn can be shown to satisfy the stationary Fokker-Planck equation
In particular, if we are considering an equilibrium (or gradient) system with f = −∇V for some potential function \(V:\mathbb {R}^{d\times n} \rightarrow \mathbb {R}\), then we have
We shall consider the case where there need not exist such a potential function, i.e., the system is not an equilibrium or gradient one. This is in fact the case for the dynamical model for savanna-forest transitions considered in this paper. Nevertheless, the invariant distribution ρn may still exist.
C.2 Macrostates and relative stability
We are typically interested in macroscopic observations of x. To this end, let us define a macrostate z = ϕn(x) where \(\phi _{n} : \mathbb {R}^{d\times n} \rightarrow \mathbb {R}^{l}\) where l is small and in particular, independent of the system size n. For example, we may take z to be the spatial average of x by setting \(z=\phi _{n}(x)\equiv \tfrac {1}{n} {\sum }_{i} x_{i}\), in which case l = 1. The invariant distribution of the stochastic process z(t) = ϕn(x(t)) is formally given by the integral
where δ is the Dirac delta function.
Our goal is come up with a notion of relative stability of different values of the macrostate z in the thermodynamic limit n →∞. In equilibrium or gradient systems, this can be done by simply defining the free energy\(F:\mathbb {R}^{l} \rightarrow \mathbb {R}\) by
Assuming this limit exists, then (45) implies pn(z) ∼ exp(−nF(z)), i.e., the most probable values of the macrostate concentrate on the minima of the free energy. Moreover, given two macrostates \(z_{1},z_{2}\in \mathbb {R}^{l}\), their relative stability can be characterized by the free energy difference: z1 is more stable than z2 if F(z1) < F(z2) and vice versa. In fact, the relative transition rates between z1 and z2 are exactly quantified by the free energy difference F(z1) − F(z2).
For non-equilibrium systems, however, the free energy (which comes from the invariant distribution) alone is insufficient to characterize relative stability. For example, the presence of non-trivial currents (say, cyclic transitions z1 → z2 → z3 → z1) may not alter the invariant distribution, but fundamentally affects transition dynamics. It turns out that if we turn to “path-space,” we may define an appropriate generalization of the free energy to non-equilibrium systems.
C.3 Free action
Fix a time horizon [0,τ] and consider the space of continuous functions \(C^{0}\left (\left [0,\tau \right ],\mathbb {R}^{d\times n}\right )\). Each path x = {x (t) : t ∈ [0,τ]} of the Langevin process (41) is a member of this space. As a point of notation, we use boldface letters to denote such paths. For a fixed initial condition x (0) = x0, the formal probability density of observing a path x is
where \(S_{n,\tau }\left [\mathbf {x}\right ] =\frac {1}{4}{\int }_{0}^{\tau }\left \Vert \dot {x}\left (t\right )-f\left (x\left (t\right )\right )\right \Vert ^{2} dt\) is the action functional (Freidlin and Wentzell 2012). As seen from (46), the path probability density is formally an “equilibrium” distribution on path space (c.f. Eq. 43), where the role of the potential function is played by Sn,τ and the state space is now all possible paths.
Now, given macrostates z1 and z2, let us define the steady-state transition probability density from z1 to z2, which can be written as the path integral
where \({\rho ^{z}_{n}}\left (\cdot \right )\) denotes the microstate invariant distribution ρn conditioned on ϕn (x) = z, i.e.,
for ϕn (x) = z and 0 otherwise.
Now, we define the free action in the thermodynamic limit
and we denote by \(Q_{n,\tau }=-\log \hat {p}_{n,\tau }\) the free action for a finite system at finite times. τn is an appropriately chosen, increasing function of n. For this and most other applications, we may take τn ∝ n.
Physically, the free action measures the rate of decay of transition probabilities as the system size n grows. According to the discussion in Appendix B, this is important in characterizing the relative stability of macrostates. In particular, to compare the relative stability of states z,w, we may define the free action difference
Then, ΔQ(z1,z2) > 0 implies the transition z1 → z2 is exponentially more likely than z2 → z1, and vice versa. Therefore, this gives us a means to identify phase transition points in non-equilibrium systems. Suppose z1 and z2 are two competing macrostate values representing two phases and the dynamics depend on some parameter w (rainfall in our case). Then, the phase transition point between z1 and z2 is exactly at the value of w for which ΔQ(z1,z2) = 0.
As a remark, it can be proved (Li and Weinan 2015) that for equilibrium systems with f = −∇V, we have ΔQ(z1,z2) = F(z1) − F(z2), i.e., the free action difference reduces to the free energy difference, and is hence a generalization of the free energy difference to non-equilibrium systems.
C.4 Numerical algorithm to compute the free action
One advantage of the free action is that it can be computed numerically even for very rare transitions. This is due to the path integral form of expression (51). Indeed, Eq. 51 has the form of a integral over a probability measure over path space, since we can write
where \(\langle \cdot \rangle _{n,\tau ,z_{1}}\) denotes expectation over a distribution in the path space C0 with formal density \(\mu _{n,\tau ,z_{1}}[\mathbf {x}]:=\delta (\phi _{n}[x(0)] - z_{1})\rho _{n}^{z_{1}}[x(0)] \hat {\rho }_{n,\tau }[\mathbf {x}] D\mathbf {x}\). Thus, we may estimate it by Markov chain Monte Carlo (MCMC) integration
where X(i), i = 1,…,N ≫ 1 is a Markov chain on the path space C0 with invariant distribution equal to \(\mu _{n,\tau ,z_{1}}\) and \(\hat {\delta }\) is an approximation to the delta function. The Markov chain can be constructed with the usual Metropolis-Hastings algorithm (Metropolis et al. 1953), but here we will require a modification that deals with high-dimensional problems (large n) using the Hamiltonian Monte Carlo method with pre-conditioning. Moreover, we use a form of thermodynamic integration similar to that in Ogata (1989) to further reduce the variance. With the value of \(\hat {p}_{n,\tau }\left (z_{1},z_{2}\right )\) obtained for large n and τ, we may use formula (49) to compute the free action. The complete algorithmic detail is described in Li and Weinan (2015) and we omit repeating it entirely here.
We note that such MCMC algorithm to compute transition rates (and hence the free action) is fundamentally different from direct simulation of the dynamical (41). The transitions in the latter may be very rare. Yet, by performing Monte Carlo moves directly on path space, we can directly sample and obtain information on these rare transition paths. We note that similar ideas has been applied to the study of chemical reactions and other rare events (Dellago et al. 1998; Bolhuis et al. 2002).
Rights and permissions
About this article
Cite this article
Li, Q., Staver, A.C., E, W. et al. Spatial feedbacks and the dynamics of savanna and forest. Theor Ecol 12, 237–262 (2019). https://doi.org/10.1007/s12080-019-0428-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-019-0428-1