Abstract
The anisotropic features of Au nanorods make them an attractive nanoscale precursor for the design of higher order nanostructured materials. However, the mode of interaction of various molecular systems on Au nanorods is not well-understood. In the present study, we have employed isothermal titration calorimetry and surface-enhanced Raman scattering for understanding various types of interactions of functional molecules on the surface of gold nanorods. The binding of thiol-bearing analyte molecules is effective with the surface of gold nanorods in acetonitrile-rich solvents and found to be weak in an aqueous medium. The effective interaction of thiol-bearing analyte molecules on nanorods is facilitated by the breakdown of cetyltrimethylammonium bromide bilayer to a monolayer in organic-rich solvent systems, thereby resulting in appreciable signals in isothermal titration calorimetry and surface-enhanced Raman spectra. The electrostatic interaction of analyte molecule is mainly driven by the charge reversal on the surface of Au nanorods on switching the solvent from aqueous to organic medium. Thus, based on isothermal titration calorimetry and surface-enhanced Raman scattering investigations, it is established that the microheterogeneous environment around the Au nanorods plays a crucial role in driving the interaction of analyte molecules.
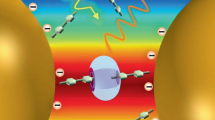
Graphical Abstract:
SYNOPSIS Cetyltrimethylammonium bromide on Au nanorod exists as a bilayer in water and as a monolayer in aqueous-organic medium, which in turn influences the surface charge and plays a crucial role in driving the substrate-analyte interactions.







Similar content being viewed by others
References
Li X, Zhu J and Wei B 2016 Hybrid Nanostructures of Metal/Two-Dimensional Nanomaterials for Plasmon-Enhanced Applications Chem. Soc. Rev. 45 3145
Thomas R, Kumar J, George J, Shanthil M, Naidu G N, Swathi R S and Thomas K G 2018 Coupling of Elementary Electronic Excitations: Drawing Parallels Between Excitons and Plasmons J. Phys. Chem. Lett. 9 919
Motl N E, Smith A F, DeSantisa C J and Skrabalak S E 2014 Engineering plasmonic metal colloids through composition and structural design Chem. Soc. Rev. 43 3823
Wu X, Xu L, Liu L, Ma W, Yin H, Kuang H, Wang L, Xu C and Kotov N A 2013 Unexpected Chirality of Nanoparticle Dimers and Ultrasensitive Chiroplasmonic Bioanalysis J. Am. Chem. Soc. 135 18629
Wu X, Hao C, Kumar J, Kuang H, Kotov N A, Liz-Marzán L M and Xu C 2018 Environmentally responsive plasmonic nanoassemblies for biosensing Chem. Soc. Rev. 47 4677
Hiromi K, Petrovykh D Y, Tarlov M J and Whitman L J 2003 Base-Dependent Competitive Adsorption of Single-Stranded DNA on Gold J. Am. Chem. Soc. 125 9014
Demers L M, Ostblom M, Zhang H, Jang N H, Liedberg B and Mirkin C A 2002 Thermal Desorption Behavior and Binding Properties of DNA Bases and Nucleosides on Gold J. Am. Chem. Soc. 124 11248
Liz-Marzán L M and Grzelczak M 2017 Growing Anisotropic Crystals at the Nanoscale Science 356 1120
Chen H, Shao L, Li Q and Wang J 2013 Gold Nanorods and their Plasmonic Properties Chem. Soc. Rev. 42 2679
Weiner R G, Kunz M R and Skrabalak S E 2015 Seeding a New Kind of Garden: Synthesis of Architecturally Defined Multimetallic Nanostructures by Seed-Mediated Co-Reduction Acc. Chem. Res. 48 2688
Baier G, Costa C, Zeller A, Baumann D, Sayer C, Araujo P H H, Mailander V, Musyanovych A and Landfester K 2011 BSA Adsorption on Differently Charged Polystyrene Nanoparticles using Isothermal Titration Calorimetry and the Influence on Cellular Uptake Macromol. Biosci. 11 628
Krishnan R and Gopidas K R 2011 \(\beta \)-Cyclodextrin as an End-to-End Connector J. Phys. Chem. Lett. 2 2094
Trani J M D, Moitessier N and Mittermaier A K 2017 Measuring Rapid Time-Scale Reaction Kinetics Using Isothermal Titration Calorimetry Anal. Chem. 89 7022
You C-C, Agasti S S and Rotello V M 2008 Isomeric Control of Protein Recognition with Amino Acid-and Dipeptide-Functionalized Gold Nanoparticles Chem. Eur. J. 14 143
Lang B 2010 Hybridization Thermodynamics of DNA Bound to Gold Nanoparticles J. Chem. Thermodyn. 42 1435
Gourishankar A, Shukla S, Ganesh K N and Sastry M 2004 Isothermal Titration Calorimetry Studies on the Binding of DNA Bases and PNA Base Monomers to Gold Nanoparticles J. Am. Chem. Soc. 126 13186
Varghese N, Vivekchand S R C, Govindaraj A and Rao C N R 2008 A calorimetric investigation of the assembly of gold nanorods to form necklaces Chem. Phys. Lett. 450 340
Moore D E, Goode D R, Seney C S and Boatwright J M 2016 Isothermal Titration Calorimetry Can Provide Critical Thinking Opportunities J. Chem. Edu. 93 304
Dam T K and Brewer C F 2002 Thermodynamic Studies of Lectin-Carbohydrate Interactions by Isothermal Titration Calorimetry Chem. Rev. 102 387
Campoy A V, Ohtaka H, Nezami A, Muzammil S and Freire E 2004 Isothermal Titration Calorimetry Curr. Protoc. Cell Biol. 23 17.8.1
Kumar J and Thomas K G 2011 Surface-Enhanced Raman Spectroscopy: Investigations at the Nanorod Edges and Dimer Junctions J. Phys. Chem. Lett. 2 610
Zrimsek A B, Chiang N, Mattei M, Zaleski S, McAnally M O, Chapman C T, Henry A-I, Schatz G C and Van Duyne R P 2017 Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy Chem. Rev. 117 7583
Nie S and Emory S R 1997 Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering Science 275 1102
Kim F, Song J H and Yang P 2002 Photochemical Synthesis of Gold Nanorods J. Am. Chem. Soc. 124 14316
Joshi H, Shirude P S, Bansal V, Ganesh K N and Sastry M 2004 Isothermal Titration Calorimetry Studies on the Binding of Amino Acids to Gold Nanoparticles J. Phys. Chem. B 108 11535
Rautaray D, Mandal S and Sastry M 2005 Synthesis of Hydroxyapatite Crystals Using Amino Acid-Capped Gold Nanoparticles as a Scaffold Langmuir 21 5185
Chao Y, Zhou Q, Li Y, Yan Y, Wu Y and Zheng J 2007 Potential Dependent Surface Enhanced Raman Scattering of 4-Mercaptopyridine on Electrochemically Roughened Silver Electrodes J. Phys. Chem. C 111 16990
Pramod P and Thomas K G 2008 Plasmon Coupling in Dimers of Au Nanorods Adv. Mater. 20 4300
Gao J, Bender C M and Murphy C J 2003 Dependence of the Gold Nanorod Aspect Ratio on the Nature of the Directing Surfactant in Aqueous Solution Langmuir 19 9065
Zheng J, Li X, Gu R and Lu T 2002 Comparison of the Surface Properties of the Assembled Silver Nanoparticle Electrode and Roughened Silver Electrode J. Phys. Chem. B 106 1019
Acknowledgements
JK acknowledges the Council of Scientific & Industrial Research (CSIR), India, for the fellowship. KGT acknowledges the Department of Science and Technology (DST Nanomission Project; SR/NM/NS-23/2016), Government of India for financial support and the J. C. Bose National Fellowship of DST.
Author information
Authors and Affiliations
Corresponding author
Additional information
$$^{\S }$$ § Dedicated to Professor M V George on the occasion of his 90 $$^\mathrm{th}$$ th Birth Anniversary.
Special Issue on Photochemistry, Photophysics and Photobiology
Rights and permissions
About this article
Cite this article
Kumar, J., Thomas, K.G. Probing the bilayer-monolayer switching of capping agents on Au nanorods and its interaction with guest molecules\(^{\S }\). J Chem Sci 130, 138 (2018). https://doi.org/10.1007/s12039-018-1550-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1550-0