Abstract
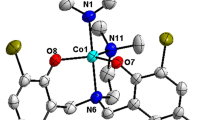
Reactions of 1-{[2-(arylazo)phenyl]iminomethyl}-2-phenol, HL [where H represents the dissociable phenolic protons of ligands and aryl groups of HL are C6H5 (HL1), p-CH3C6H5 (HL2), p-ClC6H5 (HL3)] with Os(H)(CO)(Br)(PPh3)3 in toluene and with Co(ClO4)3.6H2O in methanol afforded new facially coordinated complexes of composition [(L)Os(CO)(Br)(PPh3)] and [(L)2Co]ClO4. The anionic ligand (L) − , binds the metal in tridentate (N, N, O) manner in [(L)Os(CO)(Br)(PPh3)] and [(L)2Co]ClO4 complexes. All the Os(II) and Co(III) complexes are diamagnetic and show characteristic 1H NMR signals and intense MLCT transitions in the visible region. The cyclic voltammograms exhibited one quasi reversible oxidative response near 0.10 V vs SCE for [(L)Os(CO)(Br)(PPh3)] and [(L)2Co]ClO4 complexes displayed serial reductive responses within − 0.06 to − 1.61 V vs SCE. Single point DFT calculation was carried out to examine the nature of redox orbitals.
Conversion of ketones to corresponding alcohols has been studied using [(L)Os(CO)(Br)(PPh3)] as catalyst.

New complexes of Os(II) and Co(III) have been synthesized where the tridentate ligands are held in facial manner about the metal centre. Redox properties of the metal complexes have been studied and nature of the redox orbitals were examined on the basis of DFT calculations. Catalytic transformation of secondary ketones to corresponding alcohols using the newly synthesized Os(II) complexes as catalyst have been outlined.







Similar content being viewed by others
References
Zhang J, Braunstein P and Andy Hor T S 2008 Organometallics 27 4277
Young C G, Malarek M S, Evans D J, Doonan C J, Wee Lin Ng V and White J M 2009 Inorg. Chem. 48 1960
Poyatos M, Mata J A, and Peris E 2009 Chem. Rev. 109 3677
Liang L-C, Cheng L-C, Tsai T-L, Hu C-H and Guo W-H 2009 Inorg. Chem. 48 5697
Mattsson J, Govindaswamy P, Renfrew A K, Dyson P J, Stepnitka P, Sqss-Fink G and Therrien B 2009 Organometallics 28 4350
Traar P, Schröckeneder A, Judmaier M E, Belaj F, Baumgartner J, Sachse A and Mösch-Zanetti N C 2010 Eur. J. Inorg. Chem. 5718
Dougan S J, Habtemariam A, McHale S E, Parsons S and Sadler P J 2008 Proc. Natl. Acad. Sci. USA 105 11628
Poyatos M, Mata J A and Peris E 2009 Chem. Rev. 109 3677
Singh P, and Singh A K 2010 Eur. J. Inorg. Chem. 4187
Peacock A F A, Habtemariam A, Fernandez R, Walland V, Fabbiani F P A, Parsons S, Aird R E, Jodrell D I and Sadler P J 2006 J. Am. Chem. Soc. 128 1739
Wirth S, Rohbogner C J, Cieslak M, Kazmierczak-Baranska J, Donevski S, Nawrot B and Lorenz I P 2010 J. Biol. Inorg. Chem. 15 429
Boardman B, Hanton M J, Rensburg H V, and Tooze R P 2006 Chem. Commun. 2289
van Engelen M C, Teunissen H T, De Vries J G, and Elsevier C J 2003 J. Mol. Catal. A: Chem. 206 185
Yin A Y, Guo X Y, Dai W-L and Fan K N 2010 Chem. Commun. 46 4348
Therrien B 2009 Coord. Chem. Rev. 253 493
Sarfraz R A, Kazia T G, Iqbalb S, Afridia H I, Jamali M K, Jalbani N and Arain M B 2008 Appl. Organometal. Chem. 22 187
Matteoli U, Menchi G, Bianchi M and Piacenti F 1988 J. Mol. Catal. 44 347
Matteoli U, Menchi G, Bianchi M, Piacenti F, Ianelli S and Nardelli M 1995 J. Organomet. Chem. 498 177
Matteoli U, Menchi G, Bianchi M and Piacenti F 1991 J. Mol. Catal. 64 257
Nomura K, Ogura H and Imanishi Y 2001 J. Mol. Catal. A: Chem. 166 345
Nomura K, Ogura H and Imanishi Y 2002 J. Mol. Catal. A: Chem. 178 105
Chaudhuri P and Wieghardt K 1987 Prog. Inorg. Chem. 35 329
Blake A J and Schröder M 1990 Adv. Inorg. Chem. 351
Spicer M D and Reglinski J 2009 Eur. J. Inorg. Chem. 1553
Iengo E, Zangrando E, Baiutti E, Munini F and Alessio E 2005 Eur. J. Inorg. Chem. 1019
St.-J. Foreman M R, Hill A F, Owen G R, White A J P and Williams D J 2003 Organometallics 22 4446
Garcia R, Paulo A and Santos I 2009 Inorg. Chim. Acta. 362 4315
Harding D J, Harding P, Kivnang J and Adams H 2010 Transition Met. Chem. 35 521
Trofimenko S 1993 Chem. Rev. 93 943
Borguet Y, Sauvage X, Zaragoza G, Demonceau A and Delaude L 2010 Organometallics 29 6675
Wang X-Y, Shi H-T, Wu F-H and Zhang Q-F 2010 J. Mol. Struct. 982 66
Trofimenko S 1986 Prog. Inorg. Chem. 34 115
Walker J M, Cox A M, Wang R and Spivak G J 2010 Organometallics 29 6121
Therrien B, Vieille-Petit L and Süss-Fink G 2005 J. Mol. Struct. 738 161
Puerta M C and Valerga P 1999 Coord. Chem. Rev. 193 977
Das C, Peng S-M, Lee G-H and Goswami S 2002 New J. Chem. 26 222
Acharyya R, Peng S-M, Lee G-H and Bhattacharya S 2003 Inorg. Chem. 42 7378
Gupta P, Butcher R J and Bhattacharya S 2003 Inorg. Chem. 42 5405
Majumder K, Peng S M and Bhattacharya S 2001 J. Chem. Soc. Dalton Trans. 284
Pratihar J L, Pattanayak P, Bhaduri S, Patra D and Chattopadhyay S 2010 J. Chem. Res. 98
Pattanayak P, Pratihar J L, Patra D, Burrows A, Mohan M and Chattopadhyay S 2010 Inorg. Chim. Acta 363 2865
Perrin D D and Armarego W L F 1988 Purification of laboratory chemicals, 3rd edn.; New York: Pergamon
Parshall G W 1974 Inorg. Synth. XV 48 and 50
Trost B M and Verhoeven T R 1982 in: Comprehensive organometallic chemistry, E Abel, F G A Stone, G Wilkinson (Eds.), vol. 8; Oxford: Pergamon Press
Pattanayak P, Pratihar J L, Patra D, Burrows A, Mohan M and Chattopadhyay S 2007 Eur. J. Inorg. Chem. 4263
Parr R G and Yang W 1989 Density functional theory of atoms and molecules; Oxford: Oxford University Press
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman Jr J R, Montgomery J A, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, ammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C, Pople J A, Gaussian 03W, revision C.02 Gaussian, Inc., Wallingford, CT, 2004.16
Becke A D 1993 J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1998 Phys. Rev. B 37 785
Schaefer A, Hube C and Ahlrichs R 1994 J. Chem. Phys. 100 5829
Schaefer A, Horn H and Ahlrichs R 1992 J. Chem. Phys. 97 2571
Sheldrick G M 1990 SHELXS-97, Göttingen, Germany: University of Göttingen
Sheldrick G M 1997 SHELXL-97, Program for the refinement of crystals structures from diffraction data, Göttingen, Germany: University of Göttingen
Patra D, Pattanayak P, Pratihar J L and Chattopadhyay S 2007 Polyhedron 26 484
Ray U, Chand B, Mostafa G, Cheng J, Lu T-H and Sinha C 2003 Polyhedron 22 2587
Pattanayak P, Pratihar J L, Patra D, Puranik V G and Chattopadhyay S 2008 Polyhedron 27 2209
Saluzzo C and Lemaire M 2002 Adv. Synt. Catal. 344 915
Harmon R E, Gupta S K and Brown D J 1973 Chem. Rev. 73 21
Pintar A and Batista J 1999 Catal. Today 53 35
Mallat T, Bodmer M and Baiker A 1997 Catal. Lett. 44 95
Venkatachalam G and Ramesh R 2005 Tetr. Lett. 46 5215
Kannan S, Ramesh R and Liu Y 2007 J. Organomet. Chem. 692 3380
Pratihar J L, Bhaduri S, Pattanayak P, Patra D and Chattopadhyay S 2009 J. Organomet Chem. 694 3401
Bianchini C, Peruzzini M, Farnetti E, Kagpar J and Graziani M 1995 J. Organomet Chem. 488 91
Clapham S E and Morris R H 2005 Organometallics 24 479
Bianchini C, Farnetti E, Graziani M, Peruzzini M and Polo A 1993 Organometallics 12 3753
Acknowledgements
Authors thank the Department of Science and Technology (DST) (New Delhi) for funding under DST-SERC (SR/S1/IC-0026/2007) project. The necessary laboratory and infrastructural facility are provided by the Department of Chemistry, University of Kalyani. The support of DST under FIST and the University Grants Communication (UGC) SAP program to the Department of Chemistry, University of Kalyani is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
PATTANAYAK, P., PATRA, D., PRATIHAR, J.L. et al. Osmium and cobalt complexes incorporating facially coordinated N,N,O donor azo-imine ligands: Redox and catalytic properties. J Chem Sci 125, 51–62 (2013). https://doi.org/10.1007/s12039-012-0342-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-012-0342-1