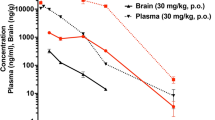
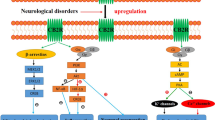
Abstract
Prolonged status epilepticus (SE) in humans causes high mortality and brain inflammation–associated neuronal injury and morbidity in survivors. Currently, the only effective treatment is to terminate the seizures swiftly to prevent brain damage. However, reliance on acute therapies alone would be imprudent due to the required short response time. Follow-on therapies that can be delivered well after the SE onset are in an urgent need. Cannabinoid receptor type 2 (CB2), a G protein-coupled receptor that can be expressed by activated brain microglia, has emerged as an appealing anti-inflammatory target for brain conditions. In the current study, we reported that the CB2 inverse agonism by our current lead compound SMM-189 largely prevented the rat primary microglia–mediated inflammation and showed moderate neuroprotection against N-methyl-d-aspartic acid (NMDA) receptor–mediated excitotoxicity in rat primary hippocampal cultures containing both neurons and glia. Using a classical mouse model of epilepsy, in which SE was induced by systemic administration of kainate (30 mg/kg, i.p.) and proceeded for 1 h, we demonstrated that SE downregulated the CB1 but slightly upregulated CB2 receptor in the hippocampus. Transient treatment with SMM-189 (6 mg/kg, i.p., b.i.d.) after the SE was interrupted by diazepam (10 mg/kg, i.p.) prevented the seizure-induced cytokine surge in the brain, neuronal death, and behavioral impairments 24 h after SE. Our results suggest that CB2 inverse agonism might provide an adjunctive anti-inflammatory therapy that can be delivered hours after SE onset, together with NMDA receptor blockers and first-line anti-convulsants, to reduce brain injury and functional deficits following prolonged seizures.








Similar content being viewed by others
References
Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, Shorvon S, Lowenstein DH (2015) A definition and classification of status epilepticus--report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 56:1515–1523. https://doi.org/10.1111/epi.13121
Betjemann JP, Lowenstein DH (2015) Status epilepticus in adults. Lancet Neurol 14:615–624. https://doi.org/10.1016/S1474-4422(15)00042-3
Rossetti AO, Lowenstein DH (2011) Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol 10:922–930. https://doi.org/10.1016/S1474-4422(11)70187-9
Annegers JF, Hauser WA, Shirts SB, Kurland LT (1987) Factors prognostic of unprovoked seizures after febrile convulsions. N Engl J Med 316:493–498. https://doi.org/10.1056/NEJM198702263160901
Tsai MH, Chuang YC, Chang HW, Chang WN, Lai SL, Huang CR, Tsai NW, Wang HC et al (2009) Factors predictive of outcome in patients with de novo status epilepticus. Qjm 102:57–62. https://doi.org/10.1093/qjmed/hcn149
Reddy DS, Kuruba R (2013) Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci 14:18284–18318. https://doi.org/10.3390/ijms140918284
Loscher W (2017) Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem Res 42:1873–1888. https://doi.org/10.1007/s11064-017-2222-z
Sanchez Fernandez I, Abend NS, Agadi S, An S, Arya R, Brenton JN, Carpenter JL, Chapman KE et al (2015) Time from convulsive status epilepticus onset to anticonvulsant administration in children. Neurology 84:2304–2311. https://doi.org/10.1212/WNL.0000000000001673
Sathe AG, Tillman H, Coles LD, Elm JJ, Silbergleit R, Chamberlain J, Kapur J, Cock HR et al (2019) Underdosing of benzodiazepines in patients with status epilepticus enrolled in established status epilepticus treatment trial. Acad Emerg Med 26:940–943. https://doi.org/10.1111/acem.13811
Trinka E, Hofler J, Leitinger M, Brigo F (2015) Pharmacotherapy for status epilepticus. Drugs 75:1499–1521. https://doi.org/10.1007/s40265-015-0454-2
Varvel NH, Jiang J, Dingledine R (2015) Candidate drug targets for prevention or modification of epilepsy. Annu Rev Pharmacol Toxicol 55:229–247. https://doi.org/10.1146/annurev-pharmtox-010814-124607
Horvath L, Fekete I, Molnar M, Valoczy R, Marton S, Fekete K (2019) The outcome of status epilepticus and long-term follow-up. Front Neurol 10:427. https://doi.org/10.3389/fneur.2019.00427
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7:31–40. https://doi.org/10.1038/nrneurol.2010.178
Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, Henshall DC, Kaufer D et al (2017) Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 58(Suppl 3):27–38. https://doi.org/10.1111/epi.13783
Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D, Brodie MJ, Brooks-Kayal AR et al (2018) Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia 59:37–66. https://doi.org/10.1111/epi.13965
Dey A, Kang X, Qiu J, Du Y, Jiang J (2016) Anti-inflammatory small molecules to treat seizures and epilepsy: from bench to bedside. Trends Pharmacol Sci 37:463–484. https://doi.org/10.1016/j.tips.2016.03.001
Schartz ND, Wyatt-Johnson SK, Price LR, Colin SA, Brewster AL (2018) Status epilepticus triggers long-lasting activation of complement C1q-C3 signaling in the hippocampus that correlates with seizure frequency in experimental epilepsy. Neurobiol Dis 109:163–173. https://doi.org/10.1016/j.nbd.2017.10.012
Nagib MM, Yu Y, Jiang J (2020) Targeting prostaglandin receptor EP2 for adjunctive treatment of status epilepticus. Pharmacol Ther:107504. https://doi.org/10.1016/j.pharmthera.2020.107504
Loscher W, Klitgaard H, Twyman RE, Schmidt D (2013) New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov 12:757–776. https://doi.org/10.1038/nrd4126
Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69:16–24. https://doi.org/10.1016/j.neuropharm.2012.04.004
Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M (2015) Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci 16:264–277. https://doi.org/10.1038/nrn3937
Stella N (2010) Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58:1017–1030. https://doi.org/10.1002/glia.20983
Baek JH, Darlington CL, Smith PF, Ashton JC (2013) Antibody testing for brain immunohistochemistry: brain immunolabeling for the cannabinoid CB(2) receptor. J Neurosci Methods 216:87–95. https://doi.org/10.1016/j.jneumeth.2013.03.021
Dhopeshwarkar A, Mackie K (2014) CB2 cannabinoid receptors as a therapeutic target-what does the future hold? Mol Pharmacol 86:430–437. https://doi.org/10.1124/mol.114.094649
Bu W, Ren H, Deng Y, Del Mar N, Guley NM, Moore BM, Honig MG, Reiner A (2016) Mild traumatic brain injury produces neuron loss that can be rescued by modulating microglial activation using a CB2 receptor inverse agonist. Front Neurosci 10:449. https://doi.org/10.3389/fnins.2016.00449
Yu Y, Nguyen DT, Jiang J (2019) G protein-coupled receptors in acquired epilepsy: druggability and translatability. Prog Neurobiol 183:101682. https://doi.org/10.1016/j.pneurobio.2019.101682
Zhang J, Chen C (2018) Alleviation of neuropathology by inhibition of monoacylglycerol lipase in APP transgenic mice lacking CB2 receptors. Mol Neurobiol 55:4802–4810. https://doi.org/10.1007/s12035-017-0689-x
Mecha M, Carrillo-Salinas FJ, Feliu A, Mestre L, Guaza C (2016) Microglia activation states and cannabinoid system: therapeutic implications. Pharmacol Ther 166:40–55. https://doi.org/10.1016/j.pharmthera.2016.06.011
Huizenga MN, Wicker E, Beck VC, Forcelli PA (2017) Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia 58:1593–1602. https://doi.org/10.1111/epi.13842
Rowley S, Sun X, Lima IV, Tavenier A, de Oliveira ACP, Dey SK, Danzer SC (2017) Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia 58:e162–e166. https://doi.org/10.1111/epi.13930
Shapiro L, Wong JC, Escayg A (2019) Reduced cannabinoid 2 receptor activity increases susceptibility to induced seizures in mice. Epilepsia 60:2359–2369. https://doi.org/10.1111/epi.16388
Presley CS, Mustafa SM, Abidi AH, Moore BM 2nd (2015) Synthesis and biological evaluation of (3′,5′-dichloro-2,6-dihydroxy-biphenyl-4-yl)-aryl/alkyl-methanone selective CB2 inverse agonist. Bioorg Med Chem 23:5390–5401. https://doi.org/10.1016/j.bmc.2015.07.057
Quan Y, Jiang J, Dingledine R (2013) EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem 288:9293–9302. https://doi.org/10.1074/jbc.M113.455816
Fu Y, Yang MS, Jiang J, Ganesh T, Joe E, Dingledine R (2015) EP2 receptor signaling regulates microglia death. Mol Pharmacol 88:161–170. https://doi.org/10.1124/mol.115.098202
Jiang J, Ganesh T, Du Y, Thepchatri P, Rojas A, Lewis I, Kurtkaya S, Li L et al (2010) Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci U S A 107:2307–2312. https://doi.org/10.1073/pnas.0909310107
Jiang J, Van TM, Ganesh T, Dingledine R (2018) Discovery of 2-piperidinyl phenyl benzamides and trisubstituted pyrimidines as positive allosteric modulators of the prostaglandin receptor EP2. ACS Chem Neurosci 9:699–707. https://doi.org/10.1021/acschemneuro.7b00486
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294. https://doi.org/10.1016/0013-4694(72)90177-0
Schauwecker PE, Steward O (1997) Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci U S A 94:4103–4108. https://doi.org/10.1073/pnas.94.8.4103
Jiang J, Yu Y, Kinjo ER, Du Y, Nguyen HP, Dingledine R (2019) Suppressing pro-inflammatory prostaglandin signaling attenuates excitotoxicity-associated neuronal inflammation and injury. Neuropharmacology 149:149–160. https://doi.org/10.1016/j.neuropharm.2019.02.011
Reiner A, Heldt SA, Presley CS, Guley NH, Elberger AJ, Deng Y, D'Surney L, Rogers JT et al (2014) Motor, visual and emotional deficits in mice after closed-head mild traumatic brain injury are alleviated by the novel CB2 inverse agonist SMM-189. Int J Mol Sci 16:758–787. https://doi.org/10.3390/ijms16010758
Guley NM, Del Mar NA, Ragsdale T, Li C, Perry AM, Moore BM, Honig MG, Reiner A (2019) Amelioration of visual deficits and visual system pathology after mild TBI with the cannabinoid type-2 receptor inverse agonist SMM-189. Exp Eye Res 182:109–124. https://doi.org/10.1016/j.exer.2019.03.013
Liu Y, McAfee SS, Guley NM, Del Mar N, Bu W, Heldt SA, Honig MG, Moore BM, 2nd, Reiner A, Heck DH (2017) Abnormalities in dynamic brain activity caused by mild traumatic brain injury are partially rescued by the cannabinoid type-2 receptor inverse agonist SMM-189. eNeuro 4. https://doi.org/10.1523/ENEURO.0387-16.2017
Kang X, Qiu J, Li Q, Bell KA, Du Y, Jung DW, Lee JY, Hao J et al (2017) Cyclooxygenase-2 contributes to oxidopamine-mediated neuronal inflammation and injury via the prostaglandin E2 receptor EP2 subtype. Sci Rep 7:9459. https://doi.org/10.1038/s41598-017-09528-z
Jiang J, Yang MS, Quan Y, Gueorguieva P, Ganesh T, Dingledine R (2015) Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis 76:126–136. https://doi.org/10.1016/j.nbd.2014.12.032
Qiu J, Li Q, Bell KA, Yao X, Du Y, Zhang E, Yu JJ, Yu Y et al (2019) Small-molecule inhibition of prostaglandin E receptor 2 impairs cyclooxygenase-associated malignant glioma growth. Br J Pharmacol 176:1680–1699. https://doi.org/10.1111/bph.14622
Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, Qui M, Speigel I, Rojas A et al (2012) Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A 109:3149–3154. https://doi.org/10.1073/pnas.1120195109
Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R (2013) Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A 110:3591–3596. https://doi.org/10.1073/pnas.1218498110
Deacon RM (2006) Assessing nest building in mice. Nat Protoc 1:1117–1119. https://doi.org/10.1038/nprot.2006.170
Roux S, Sable E, Porsolt RD (2005) Primary observation (Irwin) test in rodents for assessing acute toxicity of a test agent and its effects on behavior and physiological function. Curr Protoc Pharmacol Chapter 10:Unit 10 10. https://doi.org/10.1002/0471141755.ph1010s27
Presley C, Abidi A, Suryawanshi S, Mustafa S, Meibohm B, Moore BM (2015) Preclinical evaluation of SMM-189, a cannabinoid receptor 2-specific inverse agonist. Pharmacol Res Perspect 3:e00159. https://doi.org/10.1002/prp2.159
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184. https://doi.org/10.1016/j.tins.2012.11.008
Cherry JD, Olschowka JA, O'Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. https://doi.org/10.1186/1742-2094-11-98
von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524:3865–3895. https://doi.org/10.1002/cne.24040
Falenski KW, Carter DS, Harrison AJ, Martin BR, Blair RE, DeLorenzo RJ (2009) Temporal characterization of changes in hippocampal cannabinoid CB(1) receptor expression following pilocarpine-induced status epilepticus. Brain Res 1262:64–72. https://doi.org/10.1016/j.brainres.2009.01.036
Wu Q, Wang H (2018) The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res 148:8–16. https://doi.org/10.1016/j.eplepsyres.2018.10.002
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13:227–232. https://doi.org/10.1038/nrg3185
Marcheselli VL, Bazan NG (1996) Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem 271:24794–24799. https://doi.org/10.1074/jbc.271.40.24794
Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, Dingledine R (2014) Cyclooxygenase-2 in epilepsy. Epilepsia 55:17–25. https://doi.org/10.1111/epi.12461
Jiang J, Dingledine R (2013) Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci 34:413–423. https://doi.org/10.1016/j.tips.2013.05.003
Hartings JA, York J, Carroll CP, Hinzman JM, Mahoney E, Krueger B, Winkler MKL, Major S et al (2017) Subarachnoid blood acutely induces spreading depolarizations and early cortical infarction. Brain 140:2673–2690. https://doi.org/10.1093/brain/awx214
Jiang J, Qiu J, Li Q, Shi Z (2017) Prostaglandin E2 signaling: alternative target for glioblastoma? Trends Cancer 3:75–78. https://doi.org/10.1016/j.trecan.2016.12.002
Qiu J, Shi Z, Jiang J (2017) Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov Today 22:148–156. https://doi.org/10.1016/j.drudis.2016.09.017
Du Y, Kemper T, Qiu J, Jiang J (2016) Defining the therapeutic time window for suppressing the inflammatory prostaglandin E2 signaling after status epilepticus. Expert Rev Neurother 16:123–130. https://doi.org/10.1586/14737175.2016.1134322
Dingledine R, Varvel NH, Dudek FE (2014) When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv Exp Med Biol 813:109–122. https://doi.org/10.1007/978-94-017-8914-1_9
Lenck-Santini PP, Scott RC (2015) Mechanisms responsible for cognitive impairment in epilepsy. Cold Spring Harb Perspect Med 5. https://doi.org/10.1101/cshperspect.a022772
Chin J, Scharfman HE (2013) Shared cognitive and behavioral impairments in epilepsy and Alzheimer’s disease and potential underlying mechanisms. Epilepsy Behav 26:343–351. https://doi.org/10.1016/j.yebeh.2012.11.040
Pineda E, Jentsch JD, Shin D, Griesbach G, Sankar R, Mazarati A (2014) Behavioral impairments in rats with chronic epilepsy suggest comorbidity between epilepsy and attention deficit/hyperactivity disorder. Epilepsy Behav 31:267–275. https://doi.org/10.1016/j.yebeh.2013.10.004
Friedman D, Devinsky O (2015) Cannabinoids in the treatment of epilepsy. N Engl J Med 373:1048–1058. https://doi.org/10.1056/NEJMra1407304
Cabral GA, Raborn ES, Griffin L, Dennis J, Marciano-Cabral F (2008) CB2 receptors in the brain: role in central immune function. Br J Pharmacol 153:240–251. https://doi.org/10.1038/sj.bjp.0707584
Magloczky Z, Toth K, Karlocai R, Nagy S, Eross L, Czirjak S, Vajda J, Rasonyi G et al (2010) Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia 51(Suppl 3):115–120. https://doi.org/10.1111/j.1528-1167.2010.02624.x
Franco R, Fernandez-Suarez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86. https://doi.org/10.1016/j.pneurobio.2015.05.003
Navarro G, Borroto-Escuela D, Angelats E, Etayo I, Reyes-Resina I, Pulido-Salgado M, Rodriguez-Perez AI, Canela EI et al (2018) Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain Behav Immun 67:139–151. https://doi.org/10.1016/j.bbi.2017.08.015
Buisseret B, Alhouayek M, Guillemot-Legris O, Muccioli GG (2019) Endocannabinoid and prostanoid crosstalk in pain. Trends Mol Med 25:882–896. https://doi.org/10.1016/j.molmed.2019.04.009
Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S (2014) Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci 35:358–367. https://doi.org/10.1016/j.tips.2014.04.006
Alhouayek M, Muccioli GG (2014) COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci 35:284–292. https://doi.org/10.1016/j.tips.2014.03.001
Funding
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants R00NS082379 (J.J.), R01NS100947 (J.J.), and R21NS109687 (J.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Tennessee Health Science Center and performed in accordance with the Guide for the Care and Use of Laboratory Animals (the Guide) from the NIH.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Y., Li, L., Nguyen, D.T. et al. Inverse Agonism of Cannabinoid Receptor Type 2 Confers Anti-inflammatory and Neuroprotective Effects Following Status Epileptics. Mol Neurobiol 57, 2830–2845 (2020). https://doi.org/10.1007/s12035-020-01923-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01923-4