Abstract
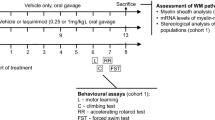
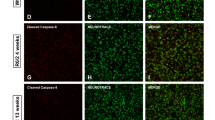
White matter (WM) abnormalities are a well-established feature of Huntington disease (HD), although their nature is not fully understood. Here, we asked whether remyelination as a measure of WM plasticity is impaired in a model of HD. Using the cuprizone assay, we examined demyelination and remyelination responses in YAC128 HD mice. Treatment with 0.2% cuprizone (CPZ) for 6 weeks resulted in significant reduction in mature (GSTπ-positive) oligodendrocyte counts and FluoroMyelin staining in the corpus callosum, leading to similar demyelination states in YAC128 and wild-type (WT) mice. Six weeks following cessation of CPZ, we observed robust remyelination in WT mice as indicated by an increase in mature oligodendrocyte counts and FluoroMyelin staining. In contrast, YAC128 mice exhibited an impaired remyelination response. The increase in mature oligodendrocyte counts in YAC128 HD mice following CPZ cessation was lower than that of WT. Furthermore, there was no increase in FluoroMyelin staining compared to the demyelinated state in YAC128 mice. We confirmed these findings using electron microscopy where the CPZ-induced reduction in myelinated axons was reversed following CPZ cessation in WT but not YAC128 mice. Our findings demonstrate that remyelination is impaired in YAC128 mice and suggest that WM plasticity may be compromised in HD.





Similar content being viewed by others
References
Nave K-A, Werner HB (2014) Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol 30:503–533. https://doi.org/10.1146/annurev-cellbio-100913-013101
Kessaris N, Pringle N, Richardson WD (2008) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philosophical Transactions of the Royal Society B: Biological Sciences 363:71–85. https://doi.org/10.1098/rstb.2006.2013
Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD (2008) PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11:1392–1401. https://doi.org/10.1038/nn.2220
Wang S, Young KM (2014) White matter plasticity in adulthood. Neuroscience 276:148–160. https://doi.org/10.1016/j.neuroscience.2013.10.018
Monje M (2018) Myelin plasticity and nervous system function. Annu Rev Neurosci 41:61–76. https://doi.org/10.1146/annurev-neuro-080317-061853
Franklin RJM, Ffrench-Constant C (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855. https://doi.org/10.1038/nrn2480
Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330:779–782. https://doi.org/10.1126/science.1190927
Walker FO (2007) Huntington’s disease. Lancet 369:218–228. https://doi.org/10.1016/S0140-6736(07)60111-1
Vonsattel JP, Myers RH, Stevens TJ et al (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Reiner A, Albin RL, Anderson KD, D'Amato CJ, Penney JB, Young AB (1988) Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A 85:5733–5737
Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL et al (2009) Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol 8:791–801. https://doi.org/10.1016/S1474-4422(09)70170-X
Paulsen JS, Nopoulos PC, Aylward E, Ross CA, Johnson H, Magnotta VA, Juhl A, Pierson RK et al (2010) Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Res Bull 82:201–207. https://doi.org/10.1016/j.brainresbull.2010.04.003
Poudel GR, Stout JC, Domínguez DJF et al (2015) Longitudinal change in white matter microstructure in Huntington’s disease: the IMAGE-HD study. Neurobiol Dis 74:406–412. https://doi.org/10.1016/j.nbd.2014.12.009
Phillips OR, Joshi SH, Squitieri F, Sanchez-Castaneda C, Narr K, Shattuck DW, Caltagirone C, Sabatini U et al (2016) Major superficial white matter abnormalities in Huntington’s disease. Front Neurosci 10:1655–1613. https://doi.org/10.3389/fnins.2016.00197
Rosas HD, Wilkens P, Salat DH, Mercaldo ND, Vangel M, Yendiki AY, Hersch SM (2018) Complex spatial and temporally defined myelin and axonal degeneration in Huntington disease. Neuroimage (Amst) 20:236–242. https://doi.org/10.1016/j.nicl.2018.01.029
Novak MJU, Seunarine KK, Gibbard CR, Hobbs NZ, Scahill RI, Clark CA, Tabrizi SJ (2014) White matter integrity in premanifest and early Huntington’s disease is related to caudate loss and disease progression. Cortex 52:98–112. https://doi.org/10.1016/j.cortex.2013.11.009
Carroll JB, Lerch JP, Franciosi S, Spreeuw A, Bissada N, Henkelman RM, Hayden MR (2011) Natural history of disease in the YAC128 mouse reveals a discrete signature of pathology in Huntington disease. Neurobiol Dis 43:257–265. https://doi.org/10.1016/j.nbd.2011.03.018
Xiang Z, Valenza M, Cui L, Leoni V, Jeong HK, Brilli E, Zhang J, Peng Q et al (2011) Peroxisome-proliferator-activated receptor gamma coactivator 1 α contributes to dysmyelination in experimental models of Huntington’s disease. J Neurosci 31:9544–9553. https://doi.org/10.1523/JNEUROSCI.1291-11.2011
Jin J, Peng Q, Hou Z, Jiang M, Wang X, Langseth AJ, Tao M, Barker PB et al (2015) Early white matter abnormalities, progressive brain pathology and motor deficits in a novel knock-in mouse model of Huntington’s disease. Hum Mol Genet 24:2508–2527. https://doi.org/10.1093/hmg/ddv016
Teo RTY, Hong X, Yu-Taeger L, Huang Y, Tan LJ, Xie Y, To XV, Guo L et al (2016) Structural and molecular myelination deficits occur prior to neuronal loss in the YAC128 and BACHD models of Huntington disease. Hum Mol Genet 25:2621–2632. https://doi.org/10.1093/hmg/ddw122
Garcia-Miralles M, Hong X, Tan LJ, Caron NS, Huang Y, To XV, Lin RY, Franciosi S et al (2016) Laquinimod rescues striatal, cortical and white matter pathology and results in modest behavioural improvements in the YAC128 model of Huntington disease. Sci Rep 6:31652. https://doi.org/10.1038/srep31652
Garcia-Miralles M, Yusof NABM, Tan JY, Radulescu CI, Sidik H, Tan LJ, Belinson H, Zach N et al (2018) Laquinimod treatment improves myelination deficits at the transcriptional and ultrastructural levels in the YAC128 mouse model of Huntington disease. Mol Neurobiol 130:1759. https://doi.org/10.1007/s12035-018-1393-1
Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N et al (2003) Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet 12:1555–1567
Pouladi MA, Morton AJ, Hayden MR (2013) Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci 14:708–721. https://doi.org/10.1038/nrn3570
Praet J, Guglielmetti C, Berneman Z, van der Linden A, Ponsaerts P (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47C:485–505. https://doi.org/10.1016/j.neubiorev.2014.10.004
Monsma PC, Brown A (2012) FluoroMyelin™ red is a bright, photostable and non-toxic fluorescent stain for live imaging of myelin. J Neurosci Methods 209:344–350. https://doi.org/10.1016/j.jneumeth.2012.06.015
Duncan ID, Marik RL, Broman AT, Heidari M (2017) Thin myelin sheaths as the hallmark of remyelination persist over time and preserve axon function. PNAS 114:E9685–E9691. https://doi.org/10.1073/pnas.1714183114
Lampron A, Larochelle A, Laflamme N, Préfontaine P, Plante MM, Sánchez MG, Yong VW, Stys PK et al (2015) Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med 212:481–495. https://doi.org/10.1084/jem.20141656
Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M et al (2013) Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136:147–167. https://doi.org/10.1093/brain/aws262
Thieben MJ, Duggins AJ, Good CD et al (2002) The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain 125:1815–1828
Reading SAJ, Yassa MA, Bakker A, Dziorny AC, Gourley LM, Yallapragada V, Rosenblatt A, Margolis RL et al (2005) Regional white matter change in pre-symptomatic Huntington’s disease: a diffusion tensor imaging study. Psychiatry Res 140:55–62. https://doi.org/10.1016/j.pscychresns.2005.05.011
Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F (2006) Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med 47:215–222
Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH (2006) Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov Disord 21:1317–1325. https://doi.org/10.1002/mds.20979
Stoffers D, Sheldon S, Kuperman JM, Goldstein J, Corey-Bloom J, Aron AR (2010) Contrasting gray and white matter changes in preclinical Huntington disease: an MRI study. Neurology 74:1208–1216. https://doi.org/10.1212/WNL.0b013e3181d8c20a
Radulescu CI, Garcia-Miralles M, Sidik H, Bardile CF, Yusof NABM, Lee HU, Ho EXP, Chu CW et al (2019) Manipulation of microbiota reveals altered callosal myelination and white matter plasticity in a model of Huntington disease. Neurobiol Dis 127:65–75. https://doi.org/10.1016/j.nbd.2019.02.011
Peters A (2002) The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 31:581–593
Marner L, Nyengaard JR, Tang Y, Pakkenberg B (2003) Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462:144–152. https://doi.org/10.1002/cne.10714
Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M et al (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 157:267–276. https://doi.org/10.1016/S0002-9440(10)64537-3
Dutta R, Trapp BD (2011) Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog Neurobiol 93:1–12. https://doi.org/10.1016/j.pneurobio.2010.09.005
Madden DJ, Bennett IJ, Song AW (2009) Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev 19:415–435. https://doi.org/10.1007/s11065-009-9113-2
Bennett IJ, Madden DJ (2014) Disconnected aging: cerebral white matter integrity and age-related differences in cognition. Neuroscience 276:187–205. https://doi.org/10.1016/j.neuroscience.2013.11.026
McKenzie IA, Ohayon D, Li H et al (2014) Motor skill learning requires active central myelination. Science 346:318–322. https://doi.org/10.1126/science.1254960
Xiao L, Ohayon D, McKenzie IA et al (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat Neurosci 19:1210–1217. https://doi.org/10.1038/nn.4351
Chang K-J, Redmond SA, Chan JR (2016) Remodeling myelination: implications for mechanisms of neural plasticity. Nat Neurosci 19:190–197. https://doi.org/10.1038/nn.4200
Huang B, Wei W, Wang G, Gaertig MA, Feng Y, Wang W, Li XJ, Li S (2015) Mutant huntingtin downregulates myelin regulatory factor-mediated myelin gene expression and affects mature oligodendrocytes. Neuron 85:1212–1226. https://doi.org/10.1016/j.neuron.2015.02.026
Saher G, Stumpf SK (2015) Cholesterol in myelin biogenesis and hypomyelinating disorders. Biochim Biophys Acta 1851:1083–1094. https://doi.org/10.1016/j.bbalip.2015.02.010
Saher G, Brügger B, Lappe-Siefke C, Möbius W, Tozawa RI, Wehr MC, Wieland F, Ishibashi S et al (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci 8:468–475. https://doi.org/10.1038/nn1426
Shankaran M, Di Paolo E, Leoni V et al (2017) Early and brain region-specific decrease of de novo cholesterol biosynthesis in Huntington’s disease: a cross-validation study in Q175 knock-in mice. Neurobiol Dis 98:66–76. https://doi.org/10.1016/j.nbd.2016.11.013
Valenza M, Marullo M, Di Paolo E et al (2015) Disruption of astrocyte-neuron cholesterol cross talk affects neuronal function in Huntington’s disease. Cell Death Differ 22:690–702. https://doi.org/10.1038/cdd.2014.162
Valenza M, Leoni V, Karasinska JM, Petricca L, Fan J, Carroll J, Pouladi MA, Fossale E et al (2010) Cholesterol defect is marked across multiple rodent models of Huntington’s disease and is manifest in astrocytes. J Neurosci 30:10844–10850. https://doi.org/10.1523/JNEUROSCI.0917-10.2010
Valenza M, Rigamonti D, Goffredo D, Zuccato C, Fenu S, Jamot L, Strand A, Tarditi A et al (2005) Dysfunction of the cholesterol biosynthetic pathway in Huntington’s disease. J Neurosci 25:9932–9939. https://doi.org/10.1523/JNEUROSCI.3355-05.2005
Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D (2006) Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127:59–69. https://doi.org/10.1016/j.cell.2006.09.015
Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ et al (2006) Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab 4:349–362. https://doi.org/10.1016/j.cmet.2006.10.004
Valenza M, Cattaneo E (2011) Emerging roles for cholesterol in Huntington’s disease. Trends Neurosci 34:474–486. https://doi.org/10.1016/j.tins.2011.06.005
Valenza M, Chen JY, Di Paolo E et al (2015) Cholesterol-loaded nanoparticles ameliorate synaptic and cognitive function in Huntington’s disease mice. EMBO Mol Med 7:1547–1564. https://doi.org/10.15252/emmm.201505413
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE et al (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 344:1252304. https://doi.org/10.1126/science.1252304
Coman I, Barbin G, Charles P, Zalc B, Lubetzki C (2005) Axonal signals in central nervous system myelination, demyelination and remyelination. J Neurol Sci 233:67–71. https://doi.org/10.1016/j.jns.2005.03.029
Piaton G, Gould RM, Lubetzki C (2010) Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. J Neurochem 114:1243–1260. https://doi.org/10.1111/j.1471-4159.2010.06831.x
Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ et al (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218. https://doi.org/10.1038/nn.3469
Claycomb KI, Johnson KM, Winokur PN, Sacino A, Crocker S (2013) Astrocyte regulation of CNS inflammation and remyelination. Brain Sci 3:1109–1127. https://doi.org/10.3390/brainsci3031109
Morfini GA, You Y-M, Pollema SL, Kaminska A, Liu K, Yoshioka K, Björkblom B, Coffey ET et al (2009) Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12:864–871. https://doi.org/10.1038/nn.2346
Khakh BS, Beaumont V, Cachope R, Munoz-Sanjuan I, Goldman SA, Grantyn R (2017) Unravelling and exploiting astrocyte dysfunction in Huntington’s disease. Trends Neurosci 40:422–437. https://doi.org/10.1016/j.tins.2017.05.002
Andre R, Carty L, Tabrizi SJ (2015) Disruption of immune cell function by mutant huntingtin in Huntington’s disease pathogenesis. Curr Opin Pharmacol 26:33–38. https://doi.org/10.1016/j.coph.2015.09.008
Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM et al (2012) Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. https://doi.org/10.1038/nature11007
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A et al (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. https://doi.org/10.1038/nature11314
Acknowledgements
We thank Maria Ericsson (Electron Microscopy Facility, Harvard Medical School) for electron microscopy and the SBIC-Nikon Imaging Centre (A*STAR, Biopolis) for confocal imaging services.
Funding
C.F.B. is supported by a Singapore International Graduate Award (SINGA) from the Agency for Science, Technology and Research (A*STAR). The work was supported by grants from the Agency for Science, Technology and Research (A*STAR) and the National University of Singapore to M.A.P.
Author information
Authors and Affiliations
Contributions
R.T.Y.T. and M.A.P. conceived and planned the study. R.T.Y.T., C.F.B., N.A.B.M.Y., C.A.K., and L.J.T. performed experiments. R.T.Y.T. and M.A.P. analyzed data. Y.L.T. provided intellectual input. R.T.Y.T., Y.L.T., and M.A.P. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
(DOCX 4917 kb)
Rights and permissions
About this article
Cite this article
Teo, R.T.Y., Ferrari Bardile, C., Tay, Y.L. et al. Impaired Remyelination in a Mouse Model of Huntington Disease. Mol Neurobiol 56, 6873–6882 (2019). https://doi.org/10.1007/s12035-019-1579-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1579-1