Abstract
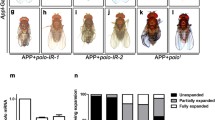
Neurodegenerative stimuli are often associated with perturbation of the axon initial segment (AIS), but it remains unclear whether AIS disruption is causative for neurodegeneration or is a downstream step in disease progression. Here, we demonstrate that either of two separate, genetically parallel pathways that disrupt the AIS induce axonal degeneration and loss of neurons in the central brain of Drosophila. Expression of a portion of the C-terminal tail of the Ank2-L isoform of Ankyrin severely shortens the AIS in Drosophila mushroom body (MB) neurons, and this shortening occurs through a mechanism that is genetically separate from the previously described Cdk5α-dependent pathway of AIS regulation. Further, either manipulation triggers morphological degeneration of MB axons and is accompanied by neuron loss. Taken together, our results are consistent with the hypothesis that disruption of the AIS is causally related to degeneration of fly central brain neurons, and we suggest that similar mechanisms may contribute to neurodegeneration in mammals.





Similar content being viewed by others
References
Rasband MN (2010) The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci 11(8):552–562. https://doi.org/10.1038/nrn2852
Palmer LM, Stuart GJ (2006) Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci 26(6):1854–1863. https://doi.org/10.1523/JNEUROSCI.4812-05.2006
Evans MD, Dumitrescu AS, Kruijssen DLH, Taylor SE, Grubb MS (2015) Rapid modulation of axon initial segment length influences repetitive spike firing. Cell Rep 13(6):1233–1245. https://doi.org/10.1016/j.celrep.2015.09.066
Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ (2008) Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci 11(2):178–186. https://doi.org/10.1038/nn2040
Khaliq ZM, Raman IM (2006) Relative contributions of axonal and somatic Na channels to action potential initiation in cerebellar Purkinje neurons. J Neurosci 26(7):1935–1944. https://doi.org/10.1523/JNEUROSCI.4664-05.2006
Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA (2008) Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A 105(22):7869–7874. https://doi.org/10.1073/pnas.0802805105
Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ et al (2012) A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell 149(5):1125–1139. https://doi.org/10.1016/j.cell.2012.03.039
Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V (1998) AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 143(5):1295–1304
Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B (2003) A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 300(5628):2091–2094. https://doi.org/10.1126/science.1085167
Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS et al (2006) A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci 26(10):2599–2613. https://doi.org/10.1523/JNEUROSCI.4314-05.2006
Wallace RH, Wang DW, Singh R, Scheffer IE, George AL Jr, Phillips HA, Saar K, Reis A et al (1998) Febrile seizures and generalized epilepsy associated with a mutation in the Na+−channel beta1 subunit gene SCN1B. Nat Genet 19(4):366–370. https://doi.org/10.1038/1252
Leussis MP, Madison JM, Petryshen TL (2012) Ankyrin 3: genetic association with bipolar disorder and relevance to disease pathophysiology. Biol Mood Anxiety Disord 2:18. https://doi.org/10.1186/2045-5380-2-18
Cruz DA, Eggan SM, Azmitia EC, Lewis DA (2004) Serotonin1A receptors at the axon initial segment of prefrontal pyramidal neurons in schizophrenia. Am J Psychiatry 161(4):739–742. https://doi.org/10.1176/appi.ajp.161.4.739
Codina-Sola M, Rodriguez-Santiago B, Homs A, Santoyo J, Rigau M, Aznar-Lain G, Del Campo M, Gener B et al (2015) Integrated analysis of whole-exome sequencing and transcriptome profiling in males with autism spectrum disorders. Mol Autism 6:21. https://doi.org/10.1186/s13229-015-0017-0
Li X, Kumar Y, Zempel H, Mandelkow E-M, Biernat J, Mandelkow E (2011) Novel diffusion barrier for axonal retention of tau in neurons and its failure in neurodegeneration. EMBO J 30(23):4825–4837
Sohn PD, Tracy TE, Son H-I, Zhou Y, Leite REP, Miller BL, Seeley WW, Grinberg LT et al (2016) Acetylated tau destabilizes the cytoskeleton in the axon initial segment and is mislocalized to the somatodendritic compartment. Mol Neurodegener 11(1):47. https://doi.org/10.1186/s13024-016-0109-0
Sanchez-Mut JV, Aso E, Panayotis N, Lott I, Dierssen M, Rabano A, Urdinguio RG, Fernandez AF et al (2013) DNA methylation map of mouse and human brain identifies target genes in Alzheimer's disease. Brain 136(Pt 10):3018–3027. https://doi.org/10.1093/brain/awt237
Marin MA, Ziburkus J, Jankowsky J, Rasband MN (2016) Amyloid-beta plaques disrupt axon initial segments. Exp Neurol 281:93–98. https://doi.org/10.1016/j.expneurol.2016.04.018
Benusa SD, George NM, Sword BA, DeVries GH, Dupree JL (2017) Acute neuroinflammation induces AIS structural plasticity in a NOX2-dependent manner. J Neuroinflammation 14(1):116. https://doi.org/10.1186/s12974-017-0889-3
Trunova S, Baek B, Giniger E (2011) Cdk5 regulates the size of an axon initial segment-like compartment in mushroom body neurons of the Drosophila central brain. J Neurosci 31(29):10451–10462. https://doi.org/10.1523/JNEUROSCI.0117-11.2011
Hortsch M (2002) The axonal localization of large Drosophila Ankyrin2 protein isoforms is essential for neuronal functionality. Mol Cell Neurosci 20(1):43–55. https://doi.org/10.1006/mcne.2002.1113
Bouley M, Tian MZ, Paisley K, Shen YC, Malhotra JD, Hortsch M (2000) The L1-type cell adhesion molecule neuroglian influences the stability of neural ankyrin in the Drosophila embryo but not its axonal localization. J Neurosci 20(12):4515–4523
Jegla T, Nguyen MM, Feng C, Goetschius DJ, Luna E, van Rossum DB, Kamel B, Pisupati A et al (2016) Bilaterian giant Ankyrins have a common evolutionary origin and play a conserved role in patterning the axon initial segment. PLoS Genet 12(12):e1006457. https://doi.org/10.1371/journal.pgen.1006457
Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW (2008) A presynaptic giant Ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron 58(2):195–209. https://doi.org/10.1016/j.neuron.2008.02.017
Stephan R, Goellner B, Moreno E, Frank CA, Hugenschmidt T, Genoud C, Aberle H, Pielage J (2015) Hierarchical microtubule organization controls axon caliber and transport and determines synaptic structure and stability. Dev Cell 33(1):5–21. https://doi.org/10.1016/j.devcel.2015.02.003
Trunova S, Giniger E (2012) Absence of the Cdk5 activator p35 causes adult-onset neurodegeneration in the central brain of Drosophila. Dis Model Mech 5(2):210–219. https://doi.org/10.1242/dmm.008847
Spurrier J, Shukla AK, McLinden K, Johnson K, Giniger E (2018) Altered expression of the Cdk5 activator-like protein, Cdk5alpha, causes neurodegeneration, in part by accelerating the rate of aging. Dis Model Mech 11(3):dmm031161. https://doi.org/10.1242/dmm.031161
Connell-Crowley L, Le Gall M, Vo DJ, Giniger E (2000) The cyclin-dependent kinase Cdk5 controls multiple aspects of axon patterning in vivo. Curr Biol 10(10):599–603
Connell-Crowley L, Vo D, Luke L, Giniger E (2007) Drosophila lacking the Cdk5 activator, p35, display defective axon guidance, age-dependent behavioral deficits and reduced lifespan. Mech Dev 124(5):341–349. https://doi.org/10.1016/j.mod.2007.02.002
Smith-Trunova S, Prithviraj R, Spurrier J, Kuzina I, Gu Q, Giniger E (2015) Cdk5 regulates developmental remodeling of mushroom body neurons in Drosophila: CDK5 regulates neuronal remodeling. Dev Dyn 244(12):1550–1563. https://doi.org/10.1002/dvdy.24350
Winckler B, Forscher P, Mellman I (1999) A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature 397(6721):698–701. https://doi.org/10.1038/17806
Nakada C, Ritchie K, Oba Y, Nakamura M, Hotta Y, Iino R, Kasai RS, Yamaguchi K et al (2003) Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol 5(7):626–632. https://doi.org/10.1038/ncb1009
Weiner AT, Seebold DY, Michael NL, Guignet M, Feng C, Follick B, Yusko BA, Wasilko NP et al (2018) Identification of proteins required for precise positioning of Apc2 in dendrites. G3 (Bethesda) 8(5):1841–1853. https://doi.org/10.1534/g3.118.200205
Dubreuil RR, Yu J (1994) Ankyrin and beta-spectrin accumulate independently of alpha-spectrin in Drosophila. Proc Natl Acad Sci U S A 91(22):10285–10289
Lambert S, Bennett V (1993) Postmitotic expression of ankyrinR and beta R-spectrin in discrete neuronal populations of the rat brain. J Neurosci 13(9):3725–3735
Kordeli E, Lambert S, Bennett V (1995) AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem 270(5):2352–2359
Otto E, Kunimoto M, McLaughlin T, Bennett V (1991) Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol 114(2):241–253
Muller HJ (1932) Further studies of the nature and causes of gene mutations. Proceedings of the 6th International Congress of Genetics:213–255
Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329(6136):219–222. https://doi.org/10.1038/329219a0
Shukla AK, Spurrier J, Kuzina I, Giniger E (2018–19) Hyperactive innate immunity causes degeneration of dopamine neurons upon altering activity of Cdk5. Cell Reports 26: 131-144. https://doi.org/10.1016/j.celrep.2018.12.025
Yamada R, Kuba H (2016) Structural and functional plasticity at the axon initial segment. Front Cell Neurosci 10:250. https://doi.org/10.3389/fncel.2016.00250
Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, Brown RH Jr, Brown H et al (2009) Axonal transport defects in neurodegenerative diseases. J Neurosci 29(41):12776–12786. https://doi.org/10.1523/JNEUROSCI.3463-09.2009
Acknowledgements
We thank each member of our lab, and also Chi-Hon Lee and Ela Serpe, for helpful discussions during the course of this work. We thank Jan Pielage, Chi-Hon Lee, Andreas Prokop, Melissa Rolls, and the Bloomington Drosophila Stock Center for fly stocks used in these experiments. We are grateful to Stephen Wincovitch of the NHGRI Cytogenetics and Microscopy Core Facility for his assistance with microscopy.
Funding
These experiments were supported by the Basic Neuroscience Program of the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Z01 NS003106.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplemental Fig. 1
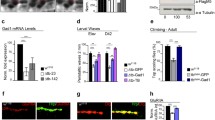
Ank2 constructs localize to soma, dendrites, and axons of MB neurons. Overexpression of the VENUS-tagged Ank2 fragments. Z-projected confocal images of late third-instar larval brains. All scale bars are equal to 20μm. MB-specific GAL4 driver 201Y is present in all samples. (a, b, c) Dorsal view of MB, illustrating expression in calyx (dendrites) and axonal lobes. (d, e, f) Posterior view of MB, revealing expression in the cell bodies. (a,d)UAS-VENUS-Ank2-S (U-A2S), (b,e)UAS-VENUS-Ank2-L4 (U-A2L4), and (c,f)UAS-VENUS-Ank2-L8 (U-A2L8) (PNG 717 kb)
High resolution image
(TIF 537 kb)
Rights and permissions
About this article
Cite this article
Spurrier, J., Shukla, A.K., Buckley, T. et al. Expression of a Fragment of Ankyrin 2 Disrupts the Structure of the Axon Initial Segment and Causes Axonal Degeneration in Drosophila. Mol Neurobiol 56, 5689–5700 (2019). https://doi.org/10.1007/s12035-019-1477-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1477-6